当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Reactivity and Site-Selectivity in Hydrogen Atom Transfer from Amino Acid C–H Bonds via Deprotonation
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.orglett.7b03948 Luca Maria Pipitone 1 , Giulia Carboni 1 , Daniela Sorrentino 1 , Marco Galeotti 1 , Michela Salamone 1 , Massimo Bietti 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.orglett.7b03948 Luca Maria Pipitone 1 , Giulia Carboni 1 , Daniela Sorrentino 1 , Marco Galeotti 1 , Michela Salamone 1 , Massimo Bietti 1
Affiliation
|
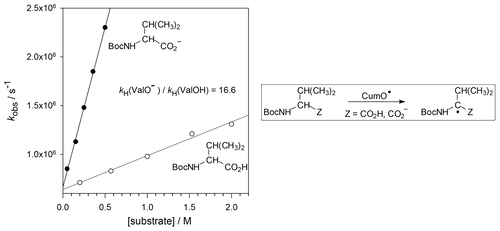
A kinetic study on the reactions of the cumyloxyl radical (CumO•) with N-Boc-protected amino acids in the presence of the strong organic base DBU has been carried out. CO2H deprotonation increases the electron density at the α-C–H bonds activating these bonds toward HAT to the electrophilic CumO• strongly influencing the intramolecular selectivity. The implications of these results are discussed in the framework of HAT-based aliphatic C–H bond functionalization of amino acids and peptides.
中文翻译:

通过去质子化从氨基酸C–H键转移氢原子的反应性和位点选择性
在强有机碱DBU的存在下,对枯草酰氧基(CumO •)与N - Boc保护的氨基酸的反应进行了动力学研究。CO 2 H去质子化增加了α-C–H键上的电子密度,从而激活了这些键对HAT的亲电性CumO •,极大地影响了分子内的选择性。这些结果的含义在基于HAT的氨基酸和肽的脂肪族C–H键功能化框架中进行了讨论。
更新日期:2018-01-12
中文翻译:

通过去质子化从氨基酸C–H键转移氢原子的反应性和位点选择性
在强有机碱DBU的存在下,对枯草酰氧基(CumO •)与N - Boc保护的氨基酸的反应进行了动力学研究。CO 2 H去质子化增加了α-C–H键上的电子密度,从而激活了这些键对HAT的亲电性CumO •,极大地影响了分子内的选择性。这些结果的含义在基于HAT的氨基酸和肽的脂肪族C–H键功能化框架中进行了讨论。











































 京公网安备 11010802027423号
京公网安备 11010802027423号