当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-Ugi Cascade Transformations for Accessing Diverse Chromenopyrrole Collections
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.orglett.7b03986 Vunnam Srinivasulu , Scott McN. Sieburth 1 , Raafat El-Awady , Noor M. Kariem , Hamadeh Tarazi , Matthew John O’Connor 2 , Taleb H. Al-Tel
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.orglett.7b03986 Vunnam Srinivasulu , Scott McN. Sieburth 1 , Raafat El-Awady , Noor M. Kariem , Hamadeh Tarazi , Matthew John O’Connor 2 , Taleb H. Al-Tel
Affiliation
|
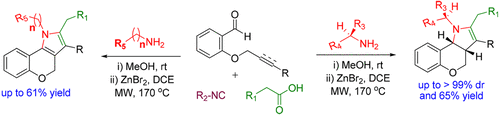
Employing a build/couple/pair strategy, a serendipitous one-pot protocol for the diastereoselective construction of diverse collections of chromenopyrroles is described. This methodology features an unprecedented five-step cascade including azomethine ylide generation followed by in situ intramolecular [3 + 2]-cycloaddition. Furthermore, this protocol was extended to access enantiopure chromenopyrroles using amino acids as chiral auxiliary. Moreover, postpairing reactions were employed to increase the diversity and complexity of our pilot compound collections.
中文翻译:

Ugi后的级联转换,用于访问各种Chromenopyrrole集合
利用构建/耦合/配对策略,描述了一个偶然的一锅协议,用于非对映选择性构建不同种类的苯并吡咯。该方法具有前所未有的五步级联反应,包括生成甲亚胺叶立德,然后进行原位分子内[3 + 2]-环加成反应。此外,该协议已扩展为使用氨基酸作为手性助剂来获得对映纯的色吡咯。此外,使用配对后反应来增加我们的试验化合物集合的多样性和复杂性。
更新日期:2018-01-12
中文翻译:

Ugi后的级联转换,用于访问各种Chromenopyrrole集合
利用构建/耦合/配对策略,描述了一个偶然的一锅协议,用于非对映选择性构建不同种类的苯并吡咯。该方法具有前所未有的五步级联反应,包括生成甲亚胺叶立德,然后进行原位分子内[3 + 2]-环加成反应。此外,该协议已扩展为使用氨基酸作为手性助剂来获得对映纯的色吡咯。此外,使用配对后反应来增加我们的试验化合物集合的多样性和复杂性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号