当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses of (−)-Tripterifordin and (−)-Neotripterifordin from Stevioside
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.joc.7b02916 Shoji Kobayashi 1 , Keisuke Shibukawa 1 , Yoshiki Hamada 1 , Takuma Kuruma 1 , Asako Kawabata 1 , Araki Masuyama 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.joc.7b02916 Shoji Kobayashi 1 , Keisuke Shibukawa 1 , Yoshiki Hamada 1 , Takuma Kuruma 1 , Asako Kawabata 1 , Araki Masuyama 1
Affiliation
|
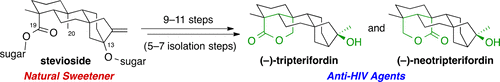
We report short syntheses of (−)-tripterifordin and (−)-neotripterifordin, potent inhibitors of HIV replication, from stevioside, a natural sweetener used worldwide. The key transformations are reduction at C13 through the formation of a tertiary chloride and subsequent three-step lactonization including a selective iodination at C20 by the photoreaction of the C19-alcohol. The title compounds were reliably obtained from stevioside in 9 and 11 steps (with 5–7 isolation steps), respectively. Additionally, the related lactone-containing ent-kaurenes, doianoterpenes A and B, and two more natural products were synthesized.
中文翻译:

甜菊糖中(-)-曲霉菌素和(-)-新三萜素的合成
我们报道了甜菊糖(全球使用的天然甜味剂)的短效合成(-)-雷公藤素和(-)-新雷公藤素,它们是HIV复制的有效抑制剂。关键的转变是通过叔氯的形成在C13还原,以及随后的三步内酯化,包括通过C19醇的光反应在C20进行选择性碘化。从甜菊糖中分别以9和11个步骤(带有5-7个分离步骤)可靠地获得了标题化合物。此外,相关的含内酯的ent- kaurenes,doianoterpenes A和B,和两个天然产品合成的。
更新日期:2018-01-12
中文翻译:

甜菊糖中(-)-曲霉菌素和(-)-新三萜素的合成
我们报道了甜菊糖(全球使用的天然甜味剂)的短效合成(-)-雷公藤素和(-)-新雷公藤素,它们是HIV复制的有效抑制剂。关键的转变是通过叔氯的形成在C13还原,以及随后的三步内酯化,包括通过C19醇的光反应在C20进行选择性碘化。从甜菊糖中分别以9和11个步骤(带有5-7个分离步骤)可靠地获得了标题化合物。此外,相关的含内酯的ent- kaurenes,doianoterpenes A和B,和两个天然产品合成的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号