Chemosphere ( IF 8.1 ) Pub Date : 2018-01-12 , DOI: 10.1016/j.chemosphere.2018.01.047 Bo Jiang , Haihong He , Yijie Liu , Yizhen Tang , Siyi Luo , Zhaohui Wang
|
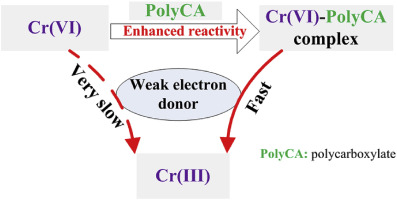
This study reports that the redox reactions between weak electron donors and Cr(VI) can be significantly accelerated by many environmentally occurring or industrially produced polycarboxylates (PolyCAs). The results demonstrate that oxalic acid (OA) can act as a redox mediator to accelerate the reduction of Cr(VI) by As(III) in pH range of 2.0–5.0, as well as a reductant donating electron for Cr(VI) reduction at pH < 4.0. Density functional theory calculation results indicate that the coordination of OA with Cr(VI) can remarkably enhance the reactivity of the Cr
O bond in HCrO4− toward oxygen atom transfer or the protonation of oxo groups during Cr(VI) reduction. Moreover, the ligand field effect can also cause instability in the tetrahedral Cr(VI) species, which probably lowers the reaction barrier in the transformation of tetrahedral Cr(VI) to octahedral Cr(III), and therefore favors the reduction of Cr(VI) to Cr(III). Similar to OA, other aliphatic and amino PolyCAs can also accelerate the reduction of Cr(VI), which depends significantly on both the electron transfer capabilities of PolyCAs and their abilities to coordinate chromium species. In general, our findings indicate the novel effect of the interplay between PolyCAs and chromium species on Cr(VI) reduction and provide significant information to develop remediation strategies for Cr(VI) contamination.中文翻译:

pH依赖的聚羧酸盐在Cr(VI)和弱电子给体之间的电子转移中的作用
这项研究报告指出,许多环境发生或工业生产的聚羧酸盐(PolyCAs)可以显着加速弱电子给体与Cr(VI)之间的氧化还原反应。结果表明,草酸(OA)可以作为氧化还原介质,在2.0–5.0的pH范围内加速As(III)对Cr(VI)的还原,并提供还原剂给电子以还原Cr(VI)在pH <4.0下 密度泛函理论计算结果表明,OA与Cr(VI)的配位可显着提高Cr的反应性
HCrO 4中的O键-Cr(VI)还原过程中向氧原子转移或羰基质子化的方向发展。此外,配体场效应还可能导致四面体Cr(VI)物种不稳定,这可能会降低四面体Cr(VI)转变为八面体Cr(III)的反应势垒,因此有利于Cr(VI)的还原)至Cr(III)。与OA相似,其他脂族和氨基PolyCA也可以加速Cr(VI)的还原,这在很大程度上取决于PolyCA的电子转移能力以及它们协调铬物种的能力。总的来说,我们的发现表明,PolyCA和铬物种之间的相互作用对Cr(VI)的还原具有新颖性,并为开发针对Cr(VI)污染的修复策略提供了重要信息。










































 京公网安备 11010802027423号
京公网安备 11010802027423号