Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-01-12 , DOI: 10.1016/j.bioorg.2018.01.018 Xu Ding , Ren-Chao Zheng , Xiao-Ling Tang , Yu-Guo Zheng
|
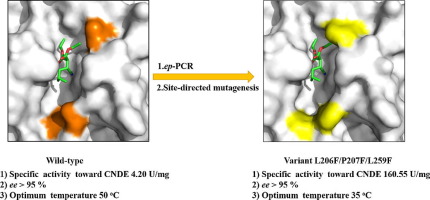
The scissile fatty acid binding site of lipases is divided into different sub-groups and plays an important role in the catalytic properties of the enzymes. In this study, the Talaromyces thermophilus lipase was engineered by altering its crevice-like binding site for efficient synthesis of chiral intermediate of Pregablin through kinetic resolution of 2-carboxyethyl-3-cyano-5-methylhexanoic acid ethyl ester (CNDE). The substitution of residues located at the crevice-like binding site with phenylalanine (Phe) resulted in significantly increased hydrolysis activity. The variant L206F/P207F/L259F exhibited a 37.23-fold and 47.02-fold improvement in the specific activity and turnover number (kcat) toward CNDE, respectively. Simultaneously, the optimum temperature and substrate preference were both altered in the variants. The study herein successfully engineered the TTL with improved catalytic properties for efficient biosynthesis of Pregablin intermediate. The investigation of structure-functional relationship provided important guidance for further modification of lipases with crevice-like binding site domain.
中文翻译:

的工程踝节菌属嗜热脂肪酶通过改变其缝隙状为手性中间体普瑞巴林的高效生物催化合成结合位点
脂肪酶的易裂脂肪酸结合位点分为不同的亚组,在酶的催化性能中起着重要的作用。在这项研究中,通过改变2-羧乙基-3-氰基-5-甲基己酸乙酯(CNDE)的动力学分辨率,通过改变缝隙状的结合位点来工程改造嗜热牛肝菌脂肪酶,从而有效地合成普瑞加布林的手性中间体。位于缝隙状结合位点的残基被苯丙氨酸(Phe)取代导致水解活性显着提高。变体L206F / P207F / L259F在比活和周转率方面表现出37.23倍和47.02倍的改善(k cat)分别指向CNDE。同时,在变体中,最佳温度和底物偏好都被改变。本文的研究成功地设计了具有改善的催化性能的TTL,以有效地合成Pregablin中间体。结构-功能关系的研究为进一步修饰具有缝隙状结合位点域的脂肪酶提供了重要指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号