Cell Host & Microbe ( IF 20.6 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.chom.2017.12.001 Casey C. Fowler , Jorge E. Galán
|
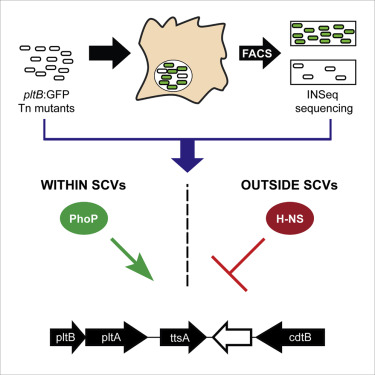
Salmonella Typhi is the cause of typhoid fever, a major global health concern. An essential virulence factor of this pathogen is typhoid toxin. In contrast to most AB-type toxins, typhoid toxin is exclusively expressed by intracellular bacteria. The regulatory networks that ensure this unique gene expression pattern are unknown. Here, we developed FAST-INSeq, a genome-wide screening approach to identify S. Typhi genes required for typhoid toxin expression within infected cells. We find that typhoid toxin expression is controlled by a silencing and counter-silencing mechanism through the opposing actions of the PhoP/PhoQ two-component regulatory system and the histone-like protein H-NS. The screen also identified bacterial mutants that alter the proportion of intracellular S. Typhi that reside within an intravacuolar environment, which was essential for toxin expression. Collectively, these data describe a regulatory mechanism that allows a bacterial pathogen to exclusively express a virulence factor when located within a specific intracellular compartment.
中文翻译:

解码控制人细胞中伤寒毒素表达的沙门氏菌调节网络
伤寒沙门氏菌是伤寒的原因,伤寒是全球主要的健康问题。该病原体的必需毒力因子是伤寒毒素。与大多数AB型毒素相反,伤寒毒素仅由细胞内细菌表达。确保这种独特的基因表达模式的调节网络是未知的。在这里,我们开发了FAST-INSeq,这是一种用于鉴定S的全基因组筛选方法。伤寒毒素在感染细胞中表达所需的伤寒基因。我们发现,伤寒毒素的表达受PhoP / PhoQ两组分调节系统和组蛋白样蛋白H-NS的相反作用的沉默和反沉默机制控制。该筛查还鉴定出了可改变细胞内S比例的细菌突变体。驻留在真空内环境中的伤寒,这对于毒素表达是必不可少的。总体而言,这些数据描述了一种调节机制,该机制使细菌病原体位于特定的细胞内腔室内时专门表达毒力因子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号