当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Structure–Activity Relationships of Inhibitors That Target the C-Terminal MEEVD on Heat Shock Protein 90
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-12-13 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00310 Marwa N. Rahimi 1 , Laura K. Buckton 1 , Samantha S. Zaiter 1 , Jessica Kho 1 , Vickie Chan 1 , Aldwin Guo 1 , Jenane Konesan 1 , SuHyeon Kwon 1 , Lok K. O. Lam 1 , Michael F. Lawler 1 , Michael Leong 1 , Gabriel D. Moldovan 1 , David A. Neale 1 , Gillian Thornton 1 , Shelli R. McAlpine 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-12-13 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00310 Marwa N. Rahimi 1 , Laura K. Buckton 1 , Samantha S. Zaiter 1 , Jessica Kho 1 , Vickie Chan 1 , Aldwin Guo 1 , Jenane Konesan 1 , SuHyeon Kwon 1 , Lok K. O. Lam 1 , Michael F. Lawler 1 , Michael Leong 1 , Gabriel D. Moldovan 1 , David A. Neale 1 , Gillian Thornton 1 , Shelli R. McAlpine 1
Affiliation
|
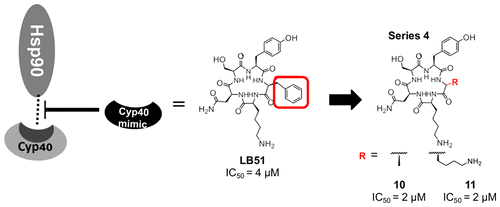
Herein, we describe the synthesis and structure–activity relationships of cyclic peptides designed to target heat shock protein 90 (Hsp90). Generating 19 compounds and evaluating their binding affinity reveals that increasing electrostatic interactions allows the compounds to bind more effectively with Hsp90 compared to the lead structure. Exchanging specific residues for lysine improves binding affinity for Hsp90, indicating some residues are not critical for interacting with the target, whereas others are essential. Replacing l- for d-amino acids produced compounds with decreased binding affinity compared to the parent structure, confirming the importance of conformation and identifying key residues most important for binding. Thus, a specific conformation and electrostatic interactions are required in order for these inhibitors to bind to Hsp90.
中文翻译:

针对热休克蛋白90的C端MEEVD的抑制剂的合成及其构效关系
在这里,我们描述了针对热休克蛋白90(Hsp90)设计的环状肽的合成和结构-活性之间的关系。生成19种化合物并评估其结合亲和力表明,与铅结构相比,增加的静电相互作用可使化合物与Hsp90更有效地结合。用赖氨酸交换特定残基可提高对Hsp90的结合亲和力,表明某些残基对于与靶标相互作用并不关键,而其他则必不可少。更换升-为d-氨基酸产生的化合物与亲本结构相比具有降低的结合亲和力,证实了构象的重要性并鉴定了对结合最重要的关键残基。因此,为了使这些抑制剂与Hsp90结合,需要特定的构象和静电相互作用。
更新日期:2017-12-13
中文翻译:

针对热休克蛋白90的C端MEEVD的抑制剂的合成及其构效关系
在这里,我们描述了针对热休克蛋白90(Hsp90)设计的环状肽的合成和结构-活性之间的关系。生成19种化合物并评估其结合亲和力表明,与铅结构相比,增加的静电相互作用可使化合物与Hsp90更有效地结合。用赖氨酸交换特定残基可提高对Hsp90的结合亲和力,表明某些残基对于与靶标相互作用并不关键,而其他则必不可少。更换升-为d-氨基酸产生的化合物与亲本结构相比具有降低的结合亲和力,证实了构象的重要性并鉴定了对结合最重要的关键残基。因此,为了使这些抑制剂与Hsp90结合,需要特定的构象和静电相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号