当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Nature and Reactivity of Ferryl Heme in Compounds I and II
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.accounts.7b00463 Peter C E Moody 1 , Emma L Raven 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.accounts.7b00463 Peter C E Moody 1 , Emma L Raven 2
Affiliation
|
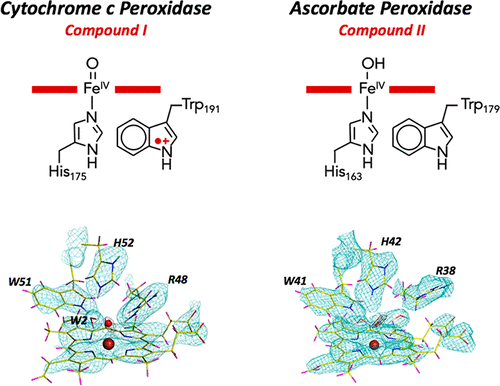
Aerobic organisms have evolved to activate oxygen from the atmosphere, which allows them to catalyze the oxidation of different kinds of substrates. This activation of oxygen is achieved by a metal center (usually iron or copper) buried within a metalloprotein. In the case of iron-containing heme enzymes, the activation of oxygen is achieved by formation of transient iron-oxo (ferryl) intermediates; these intermediates are called Compound I and Compound II. The Compound I and II intermediates were first discovered in the 1930s in horseradish peroxidase, and it is now known that these same species are used across the family of heme enzymes, which include all of the peroxidases, the heme catalases, the P450s, cytochrome c oxidase, and NO synthase. Many years have passed since the first observations, but establishing the chemical nature of these transient ferryl species remains a fundamental question that is relevant to the reactivity, and therefore the usefulness, of these species in biology.
中文翻译:

化合物 I 和 II 中 Ferryl 血红素的性质和反应性
好氧生物已经进化到可以激活大气中的氧气,这使得它们能够催化不同种类底物的氧化。这种氧的活化是通过埋在金属蛋白中的金属中心(通常是铁或铜)来实现的。在含铁血红素酶的情况下,氧的活化是通过形成瞬时的铁-氧代(ferryl)中间体来实现的;这些中间体称为化合物I和化合物II。化合物 I 和 II 中间体于 1930 年代首次在辣根过氧化物酶中发现,现在已知这些相同的物种用于血红素酶家族,包括所有过氧化物酶、血红素过氧化氢酶、P450s、细胞色素c氧化酶和一氧化氮合酶。自第一次观察以来已经过去了很多年,但确定这些短暂的ferryl物种的化学性质仍然是一个与这些物种在生物学中的反应性以及因此有用性相关的基本问题。
更新日期:2018-01-12
中文翻译:

化合物 I 和 II 中 Ferryl 血红素的性质和反应性
好氧生物已经进化到可以激活大气中的氧气,这使得它们能够催化不同种类底物的氧化。这种氧的活化是通过埋在金属蛋白中的金属中心(通常是铁或铜)来实现的。在含铁血红素酶的情况下,氧的活化是通过形成瞬时的铁-氧代(ferryl)中间体来实现的;这些中间体称为化合物I和化合物II。化合物 I 和 II 中间体于 1930 年代首次在辣根过氧化物酶中发现,现在已知这些相同的物种用于血红素酶家族,包括所有过氧化物酶、血红素过氧化氢酶、P450s、细胞色素c氧化酶和一氧化氮合酶。自第一次观察以来已经过去了很多年,但确定这些短暂的ferryl物种的化学性质仍然是一个与这些物种在生物学中的反应性以及因此有用性相关的基本问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号