International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2018-01-12 , DOI: 10.1016/j.ijhydene.2017.12.066 Junichiro Otomo , Mitsuo Koshi , Teruo Mitsumori , Hiroshi Iwasaki , Koichi Yamada
|
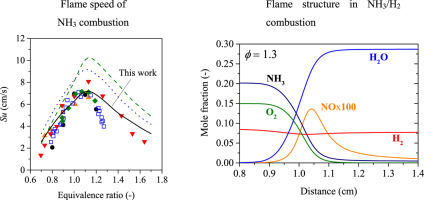
To achieve comprehensive prediction of ammonia combustion in terms of flame speed and ignition delay time, an improved mechanism of ammonia oxidation was proposed in this work. The present model (UT-LCS) was based on a previous work [Song et al., 2016] and improved by relevant elementary reactions including NH2, HNO, and N2H2. The model clearly explained reported values of laminar flame speed and ignition delay time in wide ranges of equivalence ratio and pressure. This suggests that NH2, HNO, and N2H2 reactivities play a key role to improve the reaction mechanism of ammonia oxidation in the present model. The model was also applied to demonstrate NH3/H2/air combustion. The present model also appropriately predicted the laminar flame speed of NH3/H2/air combustion as a function of equivalence ratio. Using the model, we discussed the reduction of NO concentration downstream and H2 formation via NH3 decomposition in NH3/H2 fuel-rich combustion. The results provide suggestions for effective combustion of NH3 for future applications.
中文翻译:

具有改进的氨/空气和氨/氢/空气燃烧反应机理的氨氧化化学动力学模型
为了从火焰速度和点火延迟时间的角度对氨燃烧进行综合预测,提出了一种改进的氨氧化机理。本模型(UT-LCS)基于先前的工作[Song等,2016],并通过相关的基本反应(包括NH 2,HNO和N 2 H 2)进行了改进。该模型清楚地解释了当量比值和压力范围内的层流火焰速度和点火延迟时间的报告值。这表明在本模型中,NH 2,HNO和N 2 H 2的反应性对于改善氨氧化的反应机理起着关键作用。该模型还用于演示NH 3 / H2 /空气燃烧。本模型还适当地预测了NH 3 / H 2 /空气燃烧的层流火焰速度作为当量比的函数。利用该模型,我们讨论了NO浓度下游和H的还原2经由形成NH 3在NH分解3 / H 2的富燃料燃烧。该结果为NH 3的有效燃烧提供了建议,以备将来应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号