当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Palladium Iodide-Catalyzed Oxidative Aminocarbonylation–Heterocyclization Approach to Functionalized Benzimidazoimidazoles
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.joc.7b03167 Lucia Veltri 1 , Salvatore V. Giofrè 2 , Perry Devo 3 , Roberto Romeo 2 , Adrian P. Dobbs 3 , Bartolo Gabriele 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acs.joc.7b03167 Lucia Veltri 1 , Salvatore V. Giofrè 2 , Perry Devo 3 , Roberto Romeo 2 , Adrian P. Dobbs 3 , Bartolo Gabriele 1
Affiliation
|
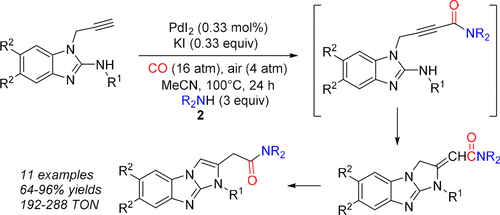
A novel carbonylative approach to the synthesis of functionalized 1H-benzo[d]imidazo[1,2-a]imidazoles is presented. The method consists of the oxidative aminocarbonylation of N-substituted-1-(prop-2-yn-1-yl)-1H-benzo[d]imidazol-2-amines, carried out in the presence of secondary nucleophilic amines, to give the corresponding alkynylamide intermediates, followed by in situ conjugated addition and double-bond isomerization, to give 2-(1-alkyl-1H-benzo[d]imidazo[1,2-a]imidazol-2-yl)acetamides. Products were obtained in good to excellent yields (64–96%) and high turnover numbers (192–288 mol of product per mol of catalyst) under relatively mild conditions (100 °C under 20 atm of a 4:1 mixture of CO–air), using a simple catalytic system, consisting of PdI2 (0.33 mol %) in conjunction with KI (0.33 equiv).
中文翻译:

碘化钯催化氧化氨基羰基化-杂环化作用的功能化苯并咪唑咪唑
提出了一种新颖的羰基合成方法,用于合成功能化的1 H-苯并[ d ]咪唑并[1,2- a ]咪唑。该方法由在仲亲核胺的存在下进行的N-取代-1-(prop-2-yn-1-yl)-1 H-苯并[ d ]咪唑-2-胺的氧化氨基羰基化反应组成。得到相应的中间体alkynylamide,随后原位偶联加法和双键异构化,以得到2-(1-烷基-1- ħ -苯并[ d ]咪唑并[1,2一]咪唑-2-基)乙酰胺。在相对温和的条件下(100°C,在20 atm的CO- 4:1混合气中,温度为100°C),获得的产品具有良好至极好的收率(64-96%)和高周转率(每摩尔催化剂192-288 mol产品)。空气),使用简单的催化系统,由PdI 2(0.33 mol%)和KI(0.33当量)组成。
更新日期:2018-01-18
中文翻译:

碘化钯催化氧化氨基羰基化-杂环化作用的功能化苯并咪唑咪唑
提出了一种新颖的羰基合成方法,用于合成功能化的1 H-苯并[ d ]咪唑并[1,2- a ]咪唑。该方法由在仲亲核胺的存在下进行的N-取代-1-(prop-2-yn-1-yl)-1 H-苯并[ d ]咪唑-2-胺的氧化氨基羰基化反应组成。得到相应的中间体alkynylamide,随后原位偶联加法和双键异构化,以得到2-(1-烷基-1- ħ -苯并[ d ]咪唑并[1,2一]咪唑-2-基)乙酰胺。在相对温和的条件下(100°C,在20 atm的CO- 4:1混合气中,温度为100°C),获得的产品具有良好至极好的收率(64-96%)和高周转率(每摩尔催化剂192-288 mol产品)。空气),使用简单的催化系统,由PdI 2(0.33 mol%)和KI(0.33当量)组成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号