当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling Hidden Mg–Mn–H Phase Relations at High Pressures and Temperatures by in Situ Synchrotron Diffraction
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02968 Kristina Spektor 1 , Wilson A. Crichton 1 , Sumit Konar 2 , Stanislav Filippov 3 , Johan Klarbring 3 , Sergei I. Simak 3 , Ulrich Häussermann 4
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02968 Kristina Spektor 1 , Wilson A. Crichton 1 , Sumit Konar 2 , Stanislav Filippov 3 , Johan Klarbring 3 , Sergei I. Simak 3 , Ulrich Häussermann 4
Affiliation
|
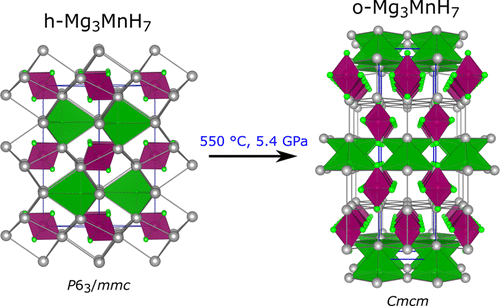
The Mg–Mn–H system was investigated by in situ high pressure studies of reaction mixtures MgH2–Mn–H2. The formation conditions of two complex hydrides with composition Mg3MnH7 were established. Previously known hexagonal Mg3MnH7 (h-Mg3MnH7) formed at pressures 1.5–2 GPa and temperatures between 480 and 500 °C, whereas an orthorhombic form (o-Mg3MnH7) was obtained at pressures above 5 GPa and temperatures above 600 °C. The crystal structures of the polymorphs feature octahedral [Mn(I)H6]5– complexes and interstitial H–. Interstitial H– is located in trigonal bipyramidal and square pyramidal interstices formed by Mg2+ ions in h- and o-Mg3MnH7, respectively. The hexagonal form can be retained at ambient pressure, whereas the orthorhombic form upon decompression undergoes a distortion to monoclinic Mg3MnH7 (m-Mg3MnH7). The structure elucidation of o- and m-Mg3MnH7 was aided by first-principles density functional theory (DFT) calculations. Calculated enthalpy versus pressure relations predict m- and o-Mg3MnH7 to be more stable than h-Mg3MnH7 above 4.3 GPa. Phonon calculations revealed o-Mg3MnH7 to be dynamically unstable at pressures below 5 GPa, which explains its phase transition to m-Mg3MnH7 on decompression. The electronic structure of the quenchable polymorphs h- and m-Mg3MnH7 is very similar. The stable 18-electron complex [MnH6]5– is mirrored in the occupied states, and calculated band gaps are around 1.5 eV. The study underlines the significance of in situ investigations for mapping reaction conditions and understanding phase relations for hydrogen-rich complex transition metal hydrides.
中文翻译:

利用原位同步加速器在高温高压下揭示隐藏的Mg-Mn-H相关系
通过对反应混合物MgH 2 -Mn-H 2进行原位高压研究,研究了Mg-Mn-H系统。建立了两种组成为Mg 3 MnH 7的复合氢化物的形成条件。先前已知的六边形Mg 3 MnH 7(h-Mg 3 MnH 7)在压力1.5–2 GPa和温度480至500°C之间形成,而正交晶形式(o-Mg 3 MnH 7)在压力高于5 GPa时形成温度高于600°C。多晶型物的晶体结构具有八面体[Mn(I)H 6 ] 5–复合物和间隙H –。间隙H –位于分别由h-Mg 3 MnH 7和o-Mg 3 MnH 7中的Mg 2+离子形成的三角锥体和方形锥体间隙中。六边形形式可以保持在环境压力下,而正交的形式在减压时经历变形为单斜晶的Mg 3 MnH 7(m-Mg 3 MnH 7)。第一原理密度泛函理论(DFT)计算有助于对o-和m-Mg 3 MnH 7的结构阐明。计算的焓与压力关系预测m-和o-Mg 3 MnH7 h比4.3 GPa以上的h-Mg 3 MnH 7更稳定。声子计算显示,o-Mg 3 MnH 7在低于5 GPa的压力下是动态不稳定的,这解释了其在减压时向m-Mg 3 MnH 7的相变。可淬灭的多晶型物h-和m-Mg 3 MnH 7的电子结构非常相似。稳定的18电子配合物[MnH 6 ] 5–在占据状态下被镜像,并且计算出的带隙约为1.5 eV。该研究强调了原位检测的意义 绘制反应条件并了解富氢复杂过渡金属氢化物的相关系的研究。
更新日期:2018-01-11
中文翻译:

利用原位同步加速器在高温高压下揭示隐藏的Mg-Mn-H相关系
通过对反应混合物MgH 2 -Mn-H 2进行原位高压研究,研究了Mg-Mn-H系统。建立了两种组成为Mg 3 MnH 7的复合氢化物的形成条件。先前已知的六边形Mg 3 MnH 7(h-Mg 3 MnH 7)在压力1.5–2 GPa和温度480至500°C之间形成,而正交晶形式(o-Mg 3 MnH 7)在压力高于5 GPa时形成温度高于600°C。多晶型物的晶体结构具有八面体[Mn(I)H 6 ] 5–复合物和间隙H –。间隙H –位于分别由h-Mg 3 MnH 7和o-Mg 3 MnH 7中的Mg 2+离子形成的三角锥体和方形锥体间隙中。六边形形式可以保持在环境压力下,而正交的形式在减压时经历变形为单斜晶的Mg 3 MnH 7(m-Mg 3 MnH 7)。第一原理密度泛函理论(DFT)计算有助于对o-和m-Mg 3 MnH 7的结构阐明。计算的焓与压力关系预测m-和o-Mg 3 MnH7 h比4.3 GPa以上的h-Mg 3 MnH 7更稳定。声子计算显示,o-Mg 3 MnH 7在低于5 GPa的压力下是动态不稳定的,这解释了其在减压时向m-Mg 3 MnH 7的相变。可淬灭的多晶型物h-和m-Mg 3 MnH 7的电子结构非常相似。稳定的18电子配合物[MnH 6 ] 5–在占据状态下被镜像,并且计算出的带隙约为1.5 eV。该研究强调了原位检测的意义 绘制反应条件并了解富氢复杂过渡金属氢化物的相关系的研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号