当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Highly Substituted Arenes via Cyclohexadiene–Alkene C–H Cross Coupling and Aromatization
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acscatal.8b00083 Anup Bhunia 1 , Armido Studer 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acscatal.8b00083 Anup Bhunia 1 , Armido Studer 1
Affiliation
|
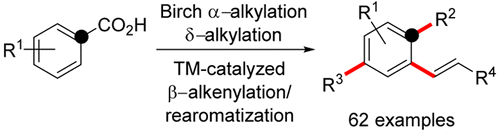
The development of a cross-coupling method for the regioselective β-alkenylation of 2,5-cyclohexadiene carboxylic acid derivatives to form ortho-alkenylarenes through in situ decarboxylation and aromatization is described. The carboxylic acid functionality is used as a traceless directing group for efficient and mild β-alkenylation. The modular sequence comprises a reductive Birch α-alkylation, ionic δ-alkylation followed by a Pd-catalyzed decarboxylative β-alkenylation with subsequent aromatization resulting in an overall three-fold ipso–para–ortho functionalization of readily accessed benzoic acid derivatives. Efficient synthesis of various alkyl-alkenylarenes under mild conditions in moderate to excellent yields is presented.
中文翻译:

通过环己二烯-烯烃C-H交叉偶联和芳构化合成高度取代的芳烃
描述了交叉偶联方法的发展,该交叉偶联方法用于2,5-环己二烯羧酸衍生物的区域选择性β-烯基化以通过原位脱羧和芳构化形成邻烯基芳烃。羧酸官能团用作有效且温和的β-烯基化反应的无痕导向基团。模块化序列包括还原桦木α烷基化,离子δ烷基化,接着通过Pd催化的脱羧β烯基化,随后芳构化导致整体三倍本位-对位-邻位容易访问的苯甲酸衍生物的官能化。提出了在温和条件下以中等至极好的收率高效合成各种烷基-烯基芳烃的方法。
更新日期:2018-01-17
中文翻译:

通过环己二烯-烯烃C-H交叉偶联和芳构化合成高度取代的芳烃
描述了交叉偶联方法的发展,该交叉偶联方法用于2,5-环己二烯羧酸衍生物的区域选择性β-烯基化以通过原位脱羧和芳构化形成邻烯基芳烃。羧酸官能团用作有效且温和的β-烯基化反应的无痕导向基团。模块化序列包括还原桦木α烷基化,离子δ烷基化,接着通过Pd催化的脱羧β烯基化,随后芳构化导致整体三倍本位-对位-邻位容易访问的苯甲酸衍生物的官能化。提出了在温和条件下以中等至极好的收率高效合成各种烷基-烯基芳烃的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号