当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Membrane Anchoring of α-Helical Proteins: Role of Tryptophan
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b11227 Alan J Situ 1 , So-Min Kang 2 , Benjamin B Frey 1 , Woojin An 3 , Chungho Kim 2 , Tobias S Ulmer 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b11227 Alan J Situ 1 , So-Min Kang 2 , Benjamin B Frey 1 , Woojin An 3 , Chungho Kim 2 , Tobias S Ulmer 1
Affiliation
|
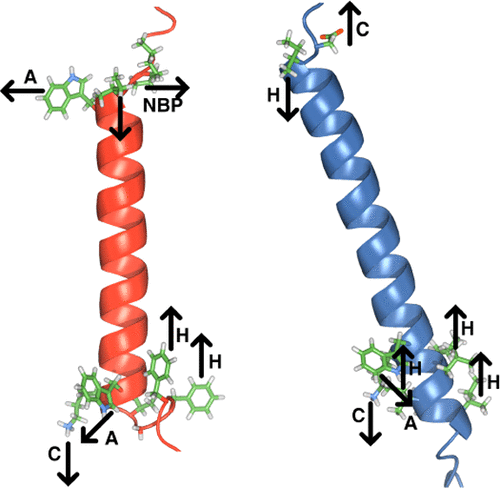
The function of membrane proteins relies on a defined orientation of protein relative to lipid. In apparent correlation to protein anchoring, tryptophan residues are enriched in the lipid headgroup region. To characterize the thermodynamic and structural basis of this relationship in α-helical membrane proteins, we examined the role of three conserved tryptophans in the folding of the heterodimeric integrin αIIbβ3 transmembrane (TM) complex in phospholipid bicelles and mammalian membranes. In the homogenous lipid environment of bicelles, tryptophan was replaceable by residues of distinct polarities. The appropriate polarity was guided by the electrostatic potential of the tryptophan surrounding, suggesting that tryptophan can complement diverse environments by adjusting the orientation of its anisotropic side chain to achieve site-specific anchoring. As a sole membrane anchor, tryptophan made a contribution of 0.4 kcal/mol to TM complex stability in bicelles. In membranes, it proved more difficult to replace tryptophan even by tyrosine, indicating a superior capacity to interact with heterogeneous lipids of biological membranes. Interestingly, at intracellular TM helix ends, where integrin activation is initiated, sequence motifs that interact with lipids via opposing polarity patterns were found to restrict TM helix orientations beyond tryptophan anchoring. In contrast to bicelles, phenylalanine became the least accepted substitute in membranes, demonstrating an increased role of the hydrophobic effect. Altogether, our study implicates a wide amphiphilic range of tryptophan, membrane complexity, and the hydrophobic effect to be important factors in tryptophan membrane anchoring.
中文翻译:

α-螺旋蛋白的膜锚定:色氨酸的作用
膜蛋白的功能依赖于蛋白质相对于脂质的确定方向。与蛋白质锚定明显相关的是,色氨酸残基在脂质头基区域中富集。为了表征α-螺旋膜蛋白中这种关系的热力学和结构基础,我们研究了三种保守色氨酸在磷脂双细胞和哺乳动物膜中异二聚体整联蛋白αIIbβ3跨膜(TM)复合物折叠中的作用。在 bicelles 的均质脂质环境中,色氨酸可以被不同极性的残基取代。适当的极性是由色氨酸周围的静电势引导的,这表明色氨酸可以通过调整其各向异性侧链的方向来补充不同的环境,以实现位点特异性锚定。作为唯一的膜锚,色氨酸对 bicelles 中 TM 复合物的稳定性贡献了 0.4 kcal/mol。在膜中,事实证明即使用酪氨酸取代色氨酸也更加困难,这表明与生物膜异质脂质相互作用的卓越能力。有趣的是,在细胞内TM螺旋末端,整合素激活被启动,通过相反极性模式与脂质相互作用的序列基序被发现限制TM螺旋方向超出色氨酸锚定。与 bicelles 相比,苯丙氨酸成为膜中最不被接受的替代品,表明疏水效应的作用增强。总而言之,我们的研究表明色氨酸的广泛两亲性、膜复杂性和疏水效应是色氨酸膜锚定的重要因素。
更新日期:2018-01-11
中文翻译:

α-螺旋蛋白的膜锚定:色氨酸的作用
膜蛋白的功能依赖于蛋白质相对于脂质的确定方向。与蛋白质锚定明显相关的是,色氨酸残基在脂质头基区域中富集。为了表征α-螺旋膜蛋白中这种关系的热力学和结构基础,我们研究了三种保守色氨酸在磷脂双细胞和哺乳动物膜中异二聚体整联蛋白αIIbβ3跨膜(TM)复合物折叠中的作用。在 bicelles 的均质脂质环境中,色氨酸可以被不同极性的残基取代。适当的极性是由色氨酸周围的静电势引导的,这表明色氨酸可以通过调整其各向异性侧链的方向来补充不同的环境,以实现位点特异性锚定。作为唯一的膜锚,色氨酸对 bicelles 中 TM 复合物的稳定性贡献了 0.4 kcal/mol。在膜中,事实证明即使用酪氨酸取代色氨酸也更加困难,这表明与生物膜异质脂质相互作用的卓越能力。有趣的是,在细胞内TM螺旋末端,整合素激活被启动,通过相反极性模式与脂质相互作用的序列基序被发现限制TM螺旋方向超出色氨酸锚定。与 bicelles 相比,苯丙氨酸成为膜中最不被接受的替代品,表明疏水效应的作用增强。总而言之,我们的研究表明色氨酸的广泛两亲性、膜复杂性和疏水效应是色氨酸膜锚定的重要因素。










































 京公网安备 11010802027423号
京公网安备 11010802027423号