当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic nucleophilic ‘umpoled’ π-allyl reagents
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7cs00449d Kim Spielmann 1, 2, 3, 4, 5 , Gilles Niel 1, 2, 3, 4, 5 , Renata Marcia de Figueiredo 1, 2, 3, 4, 5 , Jean-Marc Campagne 1, 2, 3, 4, 5
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7cs00449d Kim Spielmann 1, 2, 3, 4, 5 , Gilles Niel 1, 2, 3, 4, 5 , Renata Marcia de Figueiredo 1, 2, 3, 4, 5 , Jean-Marc Campagne 1, 2, 3, 4, 5
Affiliation
|
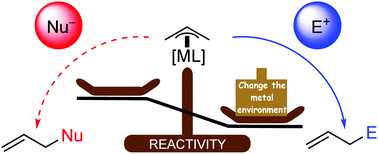
After seminal Tsuji–Trost reactions (palladium catalyzed allylation of nucleophiles via π-allyl intermediates as electrophiles), the idea of reversal reactivity of π-allyl intermediates (i.e. π-allyl as nucleophiles) has been stated since the eighties. Thanks to different transition metal sources and the modification of their electronic environment through the use of additives and ligands, such ‘reactivity switch’ of π-allyl intermediates proved its powerfulness allowing high control in regio-, diastereo- and enantio-selectivities. These methodologies have thus emerged as efficient methods in the catalytic enantioselective allylation of carbonyl compounds and imines with a deep impact on natural product and/or drug elaboration. This tutorial review highlights the concept of ‘umpoled’ reactivity of π-allyl intermediates, relying on selected recent examples.
中文翻译:

催化亲核性的“聚合型”π-烯丙基试剂
经过开创性的Tsuji–Trost反应(钯通过π-烯丙基中间体作为亲电体催化亲核试剂的烯丙基化)后,提出了π-烯丙基中间体的逆反应性的构想(即自八十年代以来就已经提出了π-烯丙基作为亲核试剂)。由于使用了不同的过渡金属源,并通过使用添加剂和配体来改变其电子环境,因此此类π-烯丙基中间体的“反应性开关”证明了其强大的功能,可以高度控制区域,非对映体和对映体选择性。因此,这些方法学已经成为羰基化合物和亚胺的催化对映选择性烯丙基化的有效方法,对天然产物和/或药物的精制具有深远的影响。本教程的回顾重点介绍了π-烯丙基中间体的“增强”反应性的概念,具体取决于最近的一些实例。
更新日期:2018-01-11
中文翻译:

催化亲核性的“聚合型”π-烯丙基试剂
经过开创性的Tsuji–Trost反应(钯通过π-烯丙基中间体作为亲电体催化亲核试剂的烯丙基化)后,提出了π-烯丙基中间体的逆反应性的构想(即自八十年代以来就已经提出了π-烯丙基作为亲核试剂)。由于使用了不同的过渡金属源,并通过使用添加剂和配体来改变其电子环境,因此此类π-烯丙基中间体的“反应性开关”证明了其强大的功能,可以高度控制区域,非对映体和对映体选择性。因此,这些方法学已经成为羰基化合物和亚胺的催化对映选择性烯丙基化的有效方法,对天然产物和/或药物的精制具有深远的影响。本教程的回顾重点介绍了π-烯丙基中间体的“增强”反应性的概念,具体取决于最近的一些实例。










































 京公网安备 11010802027423号
京公网安备 11010802027423号