当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of the a-C3H5 + O2 reaction, investigated by photoionization using synchrotron radiation†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7cp07893e D. Schleier 1, 2, 3, 4 , P. Constantinidis 1, 2, 3, 4 , N. Faßheber 4, 5, 6, 7 , I. Fischer 1, 2, 3, 4 , G. Friedrichs 4, 5, 6, 7 , P. Hemberger 8, 9, 10, 11 , E. Reusch 1, 2, 3, 4 , B. Sztáray 12, 13, 14, 15 , K. Voronova 12, 13, 14, 15
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7cp07893e D. Schleier 1, 2, 3, 4 , P. Constantinidis 1, 2, 3, 4 , N. Faßheber 4, 5, 6, 7 , I. Fischer 1, 2, 3, 4 , G. Friedrichs 4, 5, 6, 7 , P. Hemberger 8, 9, 10, 11 , E. Reusch 1, 2, 3, 4 , B. Sztáray 12, 13, 14, 15 , K. Voronova 12, 13, 14, 15
Affiliation
|
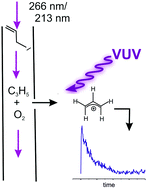
The kinetics of the combustion-relevant reaction of the allyl radical, a-C3H5, with molecular oxygen has been studied in a flow tube reactor at the vacuum ultraviolet (VUV) beamline of the Swiss Light Source storage ring, using the CRF-PEPICO (Combustion Reactions Followed by Photoelectron Photoion Coincidence Spectroscopy) setup. The ability to measure threshold photoelectron spectra enables a background-free detection of reactive species as well as an isomer-specific analysis of reaction products. Allyl was generated by direct photodissociation of allyl iodide at 266 nm and 213 nm and indirectly by the reaction of propene with Cl atoms, which were generated by photolysis from oxalyl chloride at 266 nm. Experiments were conducted at room temperature at low pressures between 0.8 and 3 mbar using Ar as the buffer gas and with excess O2 to maintain nearly pseudo-first-order reaction conditions. Whereas allyl was detected by photoionisation using synchrotron radiation, the main reaction product allyl peroxy was not observed due to dissociative ionisation of this weakly bound species. From the concentration–time profiles of the allyl signal, second-order rate constants between 1.35 × 1011 cm3 mol−1 s−1 at 0.8 mbar and 1.75 × 1011 cm3 mol−1 s−1 at 3 mbar were determined. The rates obtained for the different allyl radical generation schemes agree well with each other, but are about a factor of 2 higher than the ones reported previously using He as a buffer gas. The discrepancy is partly attributed to the higher collision efficiency of Ar causing a varying fall-off behavior. When allyl is produced by the reaction of propene with Cl atom, an unexpected product is observed at m/z = 68, which was identified as 1,3-butadienal in the threshold photoelectron spectrum. It is formed in a secondary reaction of allyl with the OCCl radical, which is generated in the 266 nm photolysis of oxalyl chloride.
中文翻译:

aC 3 H 5 + O 2反应的动力学,使用同步加速器辐射通过光电离进行研究†
烯丙基aC 3 H 5的与燃烧有关的反应动力学使用CRF-PEPICO(燃烧反应后进行光电子光子重合光谱法)设置,已经在瑞士光源存储环的真空紫外线(VUV)光束线的流管式反应器中研究了分子氧。测量阈值光电子能谱的能力使得能够在无背景的情况下检测反应性物种以及对反应产物进行异构体特异性分析。烯丙基是由烯丙基碘在266 nm和213 nm处直接光解而生成的,而丙烯与Cl原子的反应则是间接生成的,丙烯与Cl原子的反应是由草酰氯在266 nm处的光解生成的。实验是在室温下于0.8至3 mbar的低压下,使用Ar作为缓冲气体和过量的O 2进行的维持近似伪一阶反应条件。尽管使用同步加速器辐射通过光电离检测到了烯丙基,但由于该弱结合物种的离解电离,未观察到主要反应产物烯丙基过氧。根据烯丙基信号的浓度-时间曲线,在0.8 mbar时的1.35×10 11 cm 3 mol -1 s -1和1.75×10 11 cm 3 mol -1 s -1的二阶速率常数确定在3 mbar下。对于不同的烯丙基自由基生成方案,获得的速率彼此非常吻合,但比以前报道的使用He作为缓冲气体的速率高约2倍。差异部分归因于Ar的较高碰撞效率,从而导致变化的脱落行为。当丙烯与Cl原子反应生成烯丙基时,在m / z = 68处观察到意外的产物,该产物在阈值光电子能谱中被鉴定为1,3-丁二烯醛。它是由烯丙基与OCCl自由基的二次反应形成的,该反应是在266 nm的草酰氯光解中产生的。
更新日期:2018-01-11
中文翻译:

aC 3 H 5 + O 2反应的动力学,使用同步加速器辐射通过光电离进行研究†
烯丙基aC 3 H 5的与燃烧有关的反应动力学使用CRF-PEPICO(燃烧反应后进行光电子光子重合光谱法)设置,已经在瑞士光源存储环的真空紫外线(VUV)光束线的流管式反应器中研究了分子氧。测量阈值光电子能谱的能力使得能够在无背景的情况下检测反应性物种以及对反应产物进行异构体特异性分析。烯丙基是由烯丙基碘在266 nm和213 nm处直接光解而生成的,而丙烯与Cl原子的反应则是间接生成的,丙烯与Cl原子的反应是由草酰氯在266 nm处的光解生成的。实验是在室温下于0.8至3 mbar的低压下,使用Ar作为缓冲气体和过量的O 2进行的维持近似伪一阶反应条件。尽管使用同步加速器辐射通过光电离检测到了烯丙基,但由于该弱结合物种的离解电离,未观察到主要反应产物烯丙基过氧。根据烯丙基信号的浓度-时间曲线,在0.8 mbar时的1.35×10 11 cm 3 mol -1 s -1和1.75×10 11 cm 3 mol -1 s -1的二阶速率常数确定在3 mbar下。对于不同的烯丙基自由基生成方案,获得的速率彼此非常吻合,但比以前报道的使用He作为缓冲气体的速率高约2倍。差异部分归因于Ar的较高碰撞效率,从而导致变化的脱落行为。当丙烯与Cl原子反应生成烯丙基时,在m / z = 68处观察到意外的产物,该产物在阈值光电子能谱中被鉴定为1,3-丁二烯醛。它是由烯丙基与OCCl自由基的二次反应形成的,该反应是在266 nm的草酰氯光解中产生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号