Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2018-01-11 , DOI: 10.1016/j.apcatb.2018.01.025 Jeroen Poissonnier , Michiel Pelckmans , Frederik Van Waes , Kristof Moonen , Bert F. Sels , Joris W. Thybaut , Guy B. Marin
|
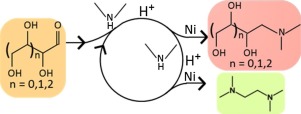
The reductive aminolysis of glucose with dimethylamine (DMA) as aminating agent has been investigated experimentally as well as via kinetic model construction. A fed-batch reactor configuration was used at following conditions: temperatures ranging between 383 K–398 K, total pressures from 6.0 MPa to 7.5 MPa, an overall catalyst to glucose ratio of 9–31 gcat mol−1, an overall DMA to glucose ratio of 12–24 mol mol−1 and an overall hydrogen to glucose ratio of 5–10 mol mol−1. Such a reactor configuration, combined with a controlled feeding rate of the sugar, allowed to significantly avoid degradation reactions. The main desired products, i.e., dimethylaminoethanol (DMAE) and tetramethylethylenediamine (TMEDA), were obtained after amination followed by retro-aldol cleavage with maximum selectivity, amounting up to 15% and 60% respectively. Retro-aldol cleavage after amination proceeds at lower temperatures, evidenced by an activation energy of 60 kJ mol−1, than without amination, where activation energies amounting to 140 kJ mol−1 have been reported. A higher catalyst to glucose ratio leads to more parallel side products such as N,N-dimethylglucamine and 4‐dimethylamino-1,2,3-butanetriol. The effects of temperature and catalyst to glucose ratio were much more pronounced than that of the total pressure or the ratio of the aminating agent to glucose. The developed kinetic model is based on the most prominent homogeneous bulk phase and heterogeneously catalyzed reactions and accounts quantitatively for degradation reactions. A statistically and physically significant model which could satisfactorily reproduce the experimental observations, was thus obtained.
中文翻译:

葡萄糖与二甲胺还原氨解反应中均相和非均相反应的动力学
已经通过实验以及通过动力学模型构建研究了以二甲胺(DMA)为胺化剂还原葡萄糖的氨解反应。在以下条件下使用分批进料反应器配置:温度介于383 K–398 K之间,总压力从6.0 MPa到7.5 MPa,催化剂与葡萄糖的总比例为9–31 g cat mol -1,葡萄糖比率为12–24 mol mol -1,总氢与葡萄糖比率为5–10 mol mol -1。这种反应器配置与糖的受控进料速率相结合,可以显着避免降解反应。胺化后,以最大选择性分别逆向羟醛裂解获得主要所需的产物,即二甲基氨基乙醇(DMAE)和四甲基乙二胺(TMEDA),分别达到15%和60%。与没有胺化的情况相比,胺化后的逆醛醇解反应在较低的温度下进行,这由60 kJ mol -1的活化能证明,据报道,没有胺化的活化能为140 kJ mol -1。较高的催化剂与葡萄糖的比率会导致更多的平行副产物,例如N,N-二甲基葡糖胺和4-二甲基氨基-1,2,3-丁三醇。温度和催化剂与葡萄糖的比率的影响比总压力或胺化剂与葡萄糖的比率的影响要明显得多。所开发的动力学模型基于最突出的均相本体相和非均相催化反应,并定量解释了降解反应。这样就获得了一个统计上和物理上有意义的模型,可以令人满意地重现实验结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号