JAMA Oncology ( IF 22.5 ) Pub Date : 2018-08-01 , DOI: 10.1001/jamaoncol.2017.4526 Yasuo Oshima 1 , Tetsuya Tanimoto 2 , Koichiro Yuji 1 , Arinobu Tojo 1
|
Importance Nivolumab and epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) are now the standard-of-care therapies in non–small cell lung cancer (NSCLC). Although EGFR-TKIs are well understood and have well-defined safety profiles, our experience with immune checkpoint inhibitors is still growing, particularly regarding the use of combinations of different classes of antitumor agents, including both the concomitant and sequential use of such agents.
Objective To determine whether nivolumab increases EGFR–TKI-associated interstitial pneumonitis (IP).
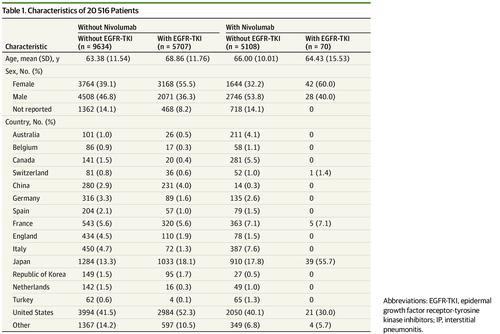
Design, Setting, and Participants A database study of 20 516 participants with NSCLC in the US Food and Drug Administration Adverse Event Reporting System (FAERS) database, performed between April 2015 and March 2017.
Main Outcomes and Measures We compared the incidence of EGFR–TKI-associated IP in patients receiving and not receiving nivolumab treatment.
Results The mean (SD) age of participants treated with EGFR-TKI, with and without nivolumab, was 64.4 (15.5) and 68.9 (11.8) years, respectively, and the proportion of men was 40.0% and 53.8%, respectively. Of the 20 516 participants with NSCLC, 985 cases (4.80%; 95% CI, 4.51-5.10) developed IP. Of 5777 patients treated with EGFR-TKI, 265 developed IP (4.59%; 95% CI, 4.06-5.16). Of 70 patients treated with both EGFR-TKI and nivolumab, 18 developed IP (25.7%; 95% CI, 16.0-37.6). The adjusted odds ratio for an interaction between EGFR-TKI and nivolumab was 4.31 (95% CI, 2.37-7.86; P < .001), suggesting the existence of an interaction. When we further stratified the patients by treatment with and without nivolumab, the odds ratio of EGFR–TKI-associated IP in cases with and without nivolumab treatment was 5.09 (95% CI, 2.87-9.03) and 1.22 (95% CI, 1.00-1.47), respectively.
Conclusions and Relevance We found a higher proportion of reports of IP for nivolumab in combination with EGFR-TKI vs treatment with either drug alone. Owing to the limitations of this study, the results warrant further confirmation. However, careful consideration should be given to the possibility of an increased risk of IP when EGFR-TKI is administered in combination with nivolumab, including concomitant and sequential use, and careful monitoring for IP is recommended.
中文翻译:

纳武利尤单抗治疗的非小细胞肺癌患者的 EGFR-TKI 相关间质性肺炎。
重要性 Nivolumab 和表皮生长因子受体酪氨酸激酶抑制剂 (EGFR-TKI) 现在是非小细胞肺癌 (NSCLC) 的标准治疗方法。尽管 EGFR-TKI 广为人知并且具有明确的安全性,但我们在免疫检查点抑制剂方面的经验仍在增长,特别是在使用不同类别的抗肿瘤药物组合方面,包括此类药物的同时使用和顺序使用。
目的 确定纳武利尤单抗是否会增加 EGFR-TKI 相关间质性肺炎 (IP)。
设计、设置和参与者 2015 年 4 月至 2017 年 3 月在美国食品和药物管理局不良事件报告系统 (FAERS) 数据库中对 20516 名 NSCLC 参与者进行的数据库研究。
主要结果和测量 我们比较了接受和未接受纳武利尤单抗治疗的患者中 EGFR-TKI 相关 IP 的发生率。
结果 接受 EGFR-TKI 治疗的参与者的平均 (SD) 年龄分别为 64.4 (15.5) 和 68.9 (11.8) 岁,男性比例分别为 40.0% 和 53.8%。在 20 516 名 NSCLC 参与者中,985 例(4.80%;95% CI,4.51-5.10)发生 IP。在接受 EGFR-TKI 治疗的 5777 名患者中,265 名发生 IP(4.59%;95% CI,4.06-5.16)。在接受 EGFR-TKI 和 nivolumab 治疗的 70 名患者中,18 名出现 IP(25.7%;95% CI,16.0-37.6)。EGFR-TKI 与 nivolumab 相互作用的调整优势比为 4.31(95% CI,2.37-7.86;P < .001),表明存在相互作用。当我们通过使用和不使用 nivolumab 治疗对患者进行进一步分层时,在使用和不使用 nivolumab 治疗的情况下,EGFR-TKI 相关 IP 的优势比为 5.09(95% CI,2.87-9.03)和 1.22(95% CI,1.00- 1.47),分别。
结论和相关性 我们发现,与单独使用任何一种药物治疗相比,nivolumab 联合 EGFR-TKI 的 IP 报告比例更高。由于本研究的局限性,结果需要进一步证实。然而,当 EGFR-TKI 与纳武利尤单抗联合使用时,应仔细考虑 IP 风险增加的可能性,包括同时和序贯使用,并建议仔细监测 IP。











































 京公网安备 11010802027423号
京公网安备 11010802027423号