JAMA Oncology ( IF 22.5 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamaoncol.2017.1617 Maud Toulmonde 1 , Nicolas Penel 2 , Julien Adam 3, 4 , Christine Chevreau 5 , Jean-Yves Blay 6 , Axel Le Cesne 7 , Emmanuelle Bompas 8 , Sophie Piperno-Neumann 9 , Sophie Cousin 1 , Thomas Grellety 1 , Thomas Ryckewaert 10 , Alban Bessede 11 , François Ghiringhelli 12 , Marina Pulido 13, 14 , Antoine Italiano 1
|
Importance There is a strong rationale for treating sarcomas with immunotherapy.
Objective To assess the efficacy and safety of programmed cell death protein 1 (PD-1) targeting in combination with metronomic chemotherapy in sarcomas.
Design, Setting, and Participants This was an open-label, multicenter, phase 2 study of 4 cohorts of patients with advanced soft-tissue sarcoma (STS), including leiomyosarcoma (LMS), undifferentiated pleomorphic sarcoma (UPS), other sarcomas (others), and gastrointestinal stromal tumor (GIST). All patients received 50 mg twice daily cyclophosphamide 1 week on and 1 week off and 200 mg of intravenous pembrolizumab every 3 weeks.
Intervention or Exposure Pembrolizumab in combination with metronomic cyclophosphamide.
Main Outcomes and Measures There was a dual primary end point, encompassing both the nonprogression and objective responses at 6 months per Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 for LMS, UPS, and others and 6-month nonprogression for GIST. An objective response rate of 20% and/or a 6-month nonprogression rate of 60% were determined as reasonable objectives for treatment with meaningful effect. Correlative studies of immune biomarkers were planned from patient tumor and plasma samples.
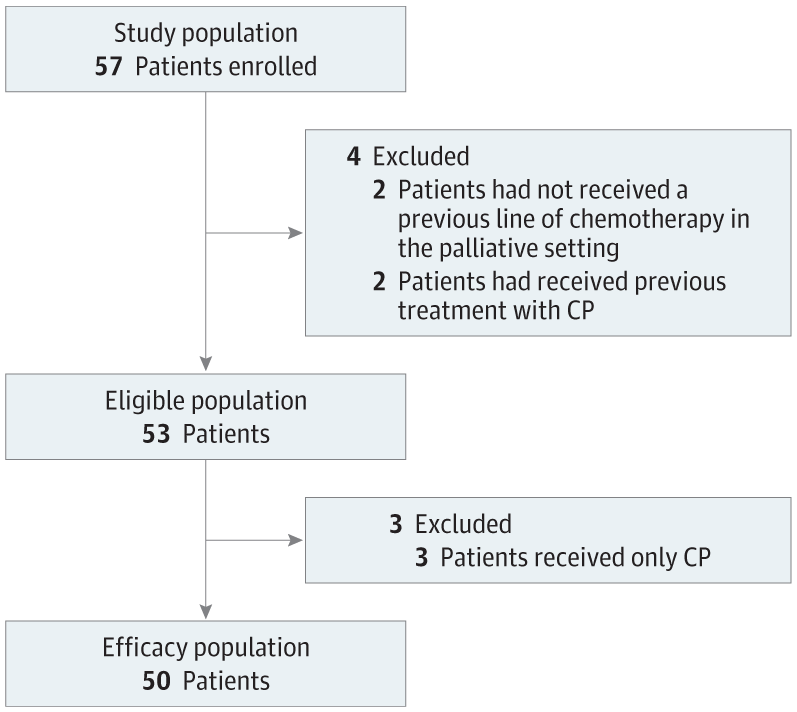
Results Between June 2015 and July 2016, 57 patients were included (median [range] age, 59.5 [18.5-84.0] years; 24 women [42%]); 50 patients were assessable for the efficacy end point. Three patients experienced tumor shrinkage, resulting in a partial response in a single solitary fibrous tumor. The 6-month nonprogression rates were 0%, 0%, 14.3% (95% CI, 1.8%-42.8%) for LMS, UPS, and others, respectively, and 11.1% (95% CI, 2.8%-48.3%) for GIST. The most frequent adverse events were grade 1 or 2 fatigue, diarrhea, and anemia. The only patient who experienced partial response was the only one with strong programmed cell death 1 ligand 1–positive staining in immune cell. Strong infiltration by macrophage expressing the inhibitory enzyme indoleamine 2,3-dioxygenase 1 (IDO1) was observed in the majority of cases. Moreover, a significant increase in the kynurenine to tryptophan ratio was observed in patient plasma samples during the study treatment.
Conclusions and Relevance We found that PD-1 inhibition has limited activity in selected STS and GIST. This may be explained by an immunosuppressive tumor microenvironment resulting from macrophage infiltration and IDO1 pathway activation.
Trial Registration clinicaltrials.gov Identifier: NCT02406781
中文翻译:

PD-1 靶向、巨噬细胞浸润和 IDO 通路激活在肉瘤 A 2 期临床试验中的应用
重要性用免疫疗法治疗肉瘤有充分的理由。
目的评估程序性细胞死亡蛋白 1(PD-1)靶向联合节拍化疗治疗肉瘤的疗效和安全性。
设计、设置和参与者这是一项开放标签、多中心、2 期研究,研究对象为 4 组晚期软组织肉瘤 (STS) 患者,包括平滑肌肉瘤 (LMS)、未分化多形性肉瘤 (UPS)、其他肉瘤(其他) )和胃肠道间质瘤(GIST)。所有患者均接受 50 mg 环磷酰胺,每日两次,连续 1 周,停药 1 周,并每 3 周静脉注射 200 mg 派姆单抗。
干预或暴露Pembrolizumab 与节拍环磷酰胺联合使用。
主要结果和措施有一个双重主要终点,包括 LMS、UPS 等的实体瘤反应评估标准 (RECIST) v1.1 在 6 个月时的无进展和客观反应以及 GIST 的 6 个月无进展。 20% 的客观缓解率和/或 60% 的 6 个月无进展率被确定为具有有意义效果的合理治疗目标。计划从患者肿瘤和血浆样本中进行免疫生物标志物的相关研究。
结果2015年6月至2016年7月期间,纳入了57名患者(中位年龄[范围],59.5[18.5-84.0]岁;24名女性[42%]); 50 名患者可评估疗效终点。三名患者经历了肿瘤缩小,导致单个孤立性纤维瘤出现部分缓解。 LMS、UPS 和其他治疗的 6 个月无进展率分别为 0%、0%、14.3%(95% CI,1.8%-42.8%)和 11.1%(95% CI,2.8%-48.3%)对于胃肠道间质瘤。最常见的不良事件是 1 级或 2 级疲劳、腹泻和贫血。唯一一位出现部分缓解的患者是唯一一位免疫细胞中程序性细胞死亡 1 配体 1 阳性染色强烈的患者。在大多数病例中观察到表达抑制酶吲哚胺 2,3-双加氧酶 1 (IDO1) 的巨噬细胞的强烈浸润。此外,在研究治疗期间,在患者血浆样本中观察到犬尿氨酸与色氨酸的比率显着增加。
结论和相关性我们发现 PD-1 抑制在选定的 STS 和 GIST 中的活性有限。这可以通过巨噬细胞浸润和 IDO1 通路激活产生的免疫抑制肿瘤微环境来解释。
试验注册ClinicalTrials.gov 标识符: NCT02406781











































 京公网安备 11010802027423号
京公网安备 11010802027423号