当前位置:
X-MOL 学术
›
Ceram. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ceramic Metal Oxides with Ni 2+ Active Phase for the Fast Degradation of Orange II Dye under Dark Ambiance
Ceramics International ( IF 5.1 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.ceramint.2018.01.071 Huihuang Chen , Julius Motuzas , Wayde Martens , João C. Diniz da Costa
Ceramics International ( IF 5.1 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.ceramint.2018.01.071 Huihuang Chen , Julius Motuzas , Wayde Martens , João C. Diniz da Costa
|
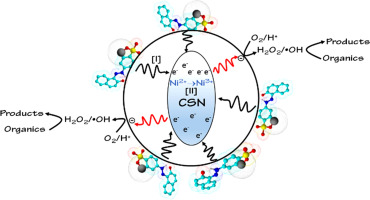
Ceramic metal oxides based on calcium strontium nickel (CSN) were synthesized via a combined EDTA-citric acid complexation method and evaluated using Orange II (OII) as the model pollutant under dark conditions, without adding any external stimulants. The CSN catalyst was characterized by a very fast reaction reaching 97% OII degradation within 5 min. The surface of CSN metal oxides proved to be very active toward the breakdown of the -N˭N- azo bond of OII. A second electron generating pathway was found as Ni phase in the CSN catalyst oxidized to Ni for the spent catalyst. Both electron generating pathways resulted in the formation of hydroxyl radicals (OH·) as determined using radical quenchers. Hydroxyl radicals were responsible for the formation of several intermediate products. The Ni phase was very active contrary to the Ni phase which could not degrade OII. The fast degradation kinetics of OII using CSN in dark at room temperature was attributed to the double electron generation pathways.
中文翻译:

具有 Ni 2+ 活性相的陶瓷金属氧化物用于在黑暗环境下快速降解橙色 II 染料
基于钙锶镍 (CSN) 的陶瓷金属氧化物通过组合 EDTA-柠檬酸络合方法合成,并在黑暗条件下使用 Orange II (OII) 作为模型污染物进行评估,不添加任何外部刺激剂。CSN 催化剂的特点是反应非常快,在 5 分钟内达到 97% 的 OII 降解。事实证明,CSN 金属氧化物的表面对 OII 的 -N˭N- 偶氮键的分解非常活跃。发现第二个电子生成途径是 CSN 催化剂中的 Ni 相氧化为 Ni 以用于废催化剂。如使用自由基猝灭剂所确定的,两种电子产生途径均导致羟基自由基(OH·)的形成。羟基自由基负责形成几种中间产物。与不能降解 OII 的 Ni 相相反,Ni 相非常活跃。在室温下在黑暗中使用 CSN 的 OII 的快速降解动力学归因于双电子生成途径。
更新日期:2018-04-01
中文翻译:

具有 Ni 2+ 活性相的陶瓷金属氧化物用于在黑暗环境下快速降解橙色 II 染料
基于钙锶镍 (CSN) 的陶瓷金属氧化物通过组合 EDTA-柠檬酸络合方法合成,并在黑暗条件下使用 Orange II (OII) 作为模型污染物进行评估,不添加任何外部刺激剂。CSN 催化剂的特点是反应非常快,在 5 分钟内达到 97% 的 OII 降解。事实证明,CSN 金属氧化物的表面对 OII 的 -N˭N- 偶氮键的分解非常活跃。发现第二个电子生成途径是 CSN 催化剂中的 Ni 相氧化为 Ni 以用于废催化剂。如使用自由基猝灭剂所确定的,两种电子产生途径均导致羟基自由基(OH·)的形成。羟基自由基负责形成几种中间产物。与不能降解 OII 的 Ni 相相反,Ni 相非常活跃。在室温下在黑暗中使用 CSN 的 OII 的快速降解动力学归因于双电子生成途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号