当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic and immunological studies on trimeric MUC1 immunodominant motif antigen-based anti-cancer vaccine candidates†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7ob02976d Mingjing Li 1, 2, 3, 4, 5 , Fan Yu 1, 2, 3, 4, 5 , Chao Yao 1, 2, 3, 4, 5 , Peng George Wang 1, 2, 3, 4, 5 , Yonghui Liu 1, 2, 3, 4, 5 , Wei Zhao 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7ob02976d Mingjing Li 1, 2, 3, 4, 5 , Fan Yu 1, 2, 3, 4, 5 , Chao Yao 1, 2, 3, 4, 5 , Peng George Wang 1, 2, 3, 4, 5 , Yonghui Liu 1, 2, 3, 4, 5 , Wei Zhao 1, 2, 3, 4, 5
Affiliation
|
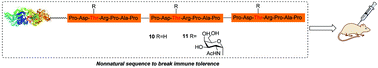
Therapeutic vaccines have been regarded as a very promising treatment modality against cancer. Tumor-associated MUC1 is a promising antigen for the design of antitumor vaccines. However, body's immune tolerance and low immunogenicity of MUC1 glycopeptides limited their use as effective antigen epitopes of therapeutic vaccines. To solve this problem, we chose the immune dominant region of MUC1 VNTRs. We designed and synthesized its linear trivalent glycopeptide fragments and coupled the fragments with BSA. Immunological evaluation indicated that the antibodies induced by glycosylated MUC1 based vaccine 11 had a stronger binding than non-glycosylated 10. The novel constructed antigen epitopes have the potential to overcome the weak immunogenicity of natural MUC1 glycopeptides and deserve further research.
中文翻译:

基于三聚体MUC1免疫优势基序抗原的抗癌疫苗候选药物的合成和免疫学研究†
治疗性疫苗被认为是一种非常有前途的抗癌治疗方法。肿瘤相关性MUC1是用于设计抗肿瘤疫苗的有前途的抗原。但是,人体的免疫耐受性和MUC1糖肽的低免疫原性限制了它们作为治疗性疫苗的有效抗原表位的用途。为了解决这个问题,我们选择了MUC1 VNTRs的免疫优势区域。我们设计并合成了其线性三价糖肽片段,并将其与BSA偶联。免疫学评估表明,基于糖基化MUC1的疫苗11诱导的抗体比未糖基化10的抗体具有更强的结合力。新型构建的抗原表位具有克服天然MUC1糖肽弱免疫原性的潜力,值得进一步研究。
更新日期:2018-01-10
中文翻译:

基于三聚体MUC1免疫优势基序抗原的抗癌疫苗候选药物的合成和免疫学研究†
治疗性疫苗被认为是一种非常有前途的抗癌治疗方法。肿瘤相关性MUC1是用于设计抗肿瘤疫苗的有前途的抗原。但是,人体的免疫耐受性和MUC1糖肽的低免疫原性限制了它们作为治疗性疫苗的有效抗原表位的用途。为了解决这个问题,我们选择了MUC1 VNTRs的免疫优势区域。我们设计并合成了其线性三价糖肽片段,并将其与BSA偶联。免疫学评估表明,基于糖基化MUC1的疫苗11诱导的抗体比未糖基化10的抗体具有更强的结合力。新型构建的抗原表位具有克服天然MUC1糖肽弱免疫原性的潜力,值得进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号