当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical-looping water splitting over ceria-modified iron oxide: performance evolution and element migration during redox cycling
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.ces.2018.01.015 Xing Zhu , Mingyue Zhang , Kongzhai Li , Yonggang Wei , Yane Zheng , Jianhang Hu , Hua Wang
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.ces.2018.01.015 Xing Zhu , Mingyue Zhang , Kongzhai Li , Yonggang Wei , Yane Zheng , Jianhang Hu , Hua Wang
|
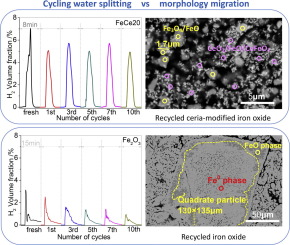
Abstract Ceria-modified iron oxide exhibits high redox performance superior to that of pure iron oxide, which can inhibit the deactivation caused by sintering of materials. In the present work, the ceria-modified iron oxide is investigated as an oxygen carrier for hydrogen production via chemical-looping water splitting process (CLWS). To identify the role of CeO2, the performance evolution and element migration of the mixed oxides during redox cycling were discussed in detail. Comparing the obvious deactivation of pure iron oxide sample, ceria-modified iron oxide shows relative constant production of hydrogen during the successive recycling water splitting. FeCe20 sample with a CeO2 mole percent of 20% shows the highest average yield of hydrogen (8651 μmol/g) with a Fe average oxygen recovery rate of 67.7% among Fe-Ce mixed oxides with different Ce molar percentage (x = 0, 10, 20 and 40%). In spite of materials sintering, the oxygen releasing/acquiring capacity and reactivity of Fe-Ce mixed oxides are three times higher than that of pure iron oxide after redox treatment. This can be ascribed to the preferable dispersion of Ce-based sub-micro particles (CeO2 and CeFeO3) and enhanced Fe-Ce interactions generated from active sites at the contact interface between iron oxides (mainly Fe3O4 particles) and Ce-based sub-micro particles (CeO2 and CeFeO3) after cycling.
中文翻译:

氧化铈改性氧化铁上的化学循环水分解:氧化还原循环过程中的性能演变和元素迁移
摘要 二氧化铈改性氧化铁具有优于纯氧化铁的高氧化还原性能,可抑制材料烧结失活。在目前的工作中,研究了氧化铈改性的氧化铁作为通过化学循环水分解工艺(CLWS)制氢的氧载体。为了确定 CeO2 的作用,详细讨论了混合氧化物在氧化还原循环过程中的性能演变和元素迁移。比较纯氧化铁样品的明显失活,氧化铈改性的氧化铁在连续循环水分解过程中显示出相对恒定的氢气产量。CeO2 摩尔百分比为 20% 的 FeCe20 样品显示出最高的平均氢气产率 (8651 μmol/g),Fe 平均氧回收率为 67。在具有不同 Ce 摩尔百分比 (x = 0、10、20 和 40%) 的 Fe-Ce 混合氧化物中占 7%。尽管进行了材料烧结,但经过氧化还原处理后,Fe-Ce 混合氧化物的放氧/吸氧能力和反应活性是纯氧化铁的三倍。这可以归因于 Ce 基亚微米颗粒(CeO2 和 CeFeO3)的优选分散以及铁氧化物(主要是 Fe3O4 颗粒)和 Ce 基亚微米颗粒接触界面处活性位点产生的增强的 Fe-Ce 相互作用。循环后的颗粒(CeO2 和 CeFeO3)。
更新日期:2018-04-01
中文翻译:

氧化铈改性氧化铁上的化学循环水分解:氧化还原循环过程中的性能演变和元素迁移
摘要 二氧化铈改性氧化铁具有优于纯氧化铁的高氧化还原性能,可抑制材料烧结失活。在目前的工作中,研究了氧化铈改性的氧化铁作为通过化学循环水分解工艺(CLWS)制氢的氧载体。为了确定 CeO2 的作用,详细讨论了混合氧化物在氧化还原循环过程中的性能演变和元素迁移。比较纯氧化铁样品的明显失活,氧化铈改性的氧化铁在连续循环水分解过程中显示出相对恒定的氢气产量。CeO2 摩尔百分比为 20% 的 FeCe20 样品显示出最高的平均氢气产率 (8651 μmol/g),Fe 平均氧回收率为 67。在具有不同 Ce 摩尔百分比 (x = 0、10、20 和 40%) 的 Fe-Ce 混合氧化物中占 7%。尽管进行了材料烧结,但经过氧化还原处理后,Fe-Ce 混合氧化物的放氧/吸氧能力和反应活性是纯氧化铁的三倍。这可以归因于 Ce 基亚微米颗粒(CeO2 和 CeFeO3)的优选分散以及铁氧化物(主要是 Fe3O4 颗粒)和 Ce 基亚微米颗粒接触界面处活性位点产生的增强的 Fe-Ce 相互作用。循环后的颗粒(CeO2 和 CeFeO3)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号