Electrochimica Acta ( IF 5.5 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.electacta.2017.12.141 Imanol Landa-Medrano , Idoia Ruiz de Larramendi , Teófilo Rojo
|
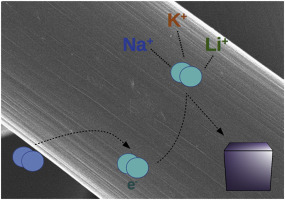
Sodium-oxygen (Na-O2) batteries have been considered as promising alternatives to lithium-oxygen batteries for high energy density applications. Herein, we report the utilisation of Li+ and K+ salts to the Na+ based electrolyte in order to tailor the oxygen reduction reaction (ORR) route in Na-O2 cells. Li+ salt addition promotes a surface confined ORR due to the incapability to stabilize superoxide radical intermediate product in the electrolyte leading to a poor electrochemical performance. The discharge capacities achieved using K+ salt additive are also lower than those obtained without it due to the incapability of KO2 to precipitate on the oxygen electrode, turning the initial solution-phase ORR to a surface-confined ORR after supersaturation. In this work we show that stabilization of the intermediate products and an efficient precipitation of the final products at the oxygen electrode are key factors governing the electrochemical performance in NaO2 batteries.
中文翻译:

通过在Na-O 2电池中添加锂盐和钾盐来修改ORR路线
钠氧(Na-O 2)电池已被认为是高能量密度应用中锂氧电池的有前途的替代品。在本文中,我们报道了Li +和K +盐对Na +基电解质的利用,以便定制Na-O 2电池中的氧还原反应(ORR)途径。Li +盐的添加由于不能稳定电解质中的超氧化物自由基中间产物而导致表面受限的ORR,从而导致不良的电化学性能。由于没有KO 2的能力,使用K +盐添加剂获得的放电容量也比没有使用K +盐添加剂获得的放电容量低。沉淀在氧电极上,过饱和后将最初的溶液相ORR变成表面受限的ORR。在这项工作中,我们表明中间产物的稳定化和最终产物在氧电极上的有效沉淀是控制Na O 2电池电化学性能的关键因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号