Electrochimica Acta ( IF 5.5 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.electacta.2018.01.045 Yu-Jen Shih , Yao-Hui Huang , C.P. Huang
|
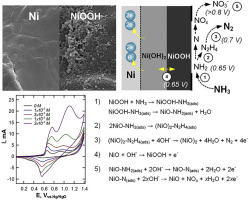
Direct electrocatalytic oxidation of ammonia was carried out using an open-pore structured nickel foam electrode via electrochemical formation of Ni(OH)2/NiOOH nano-flowers (theophrastite phase) on the nickel substrate at specific overpotentials. The electrode surface was analyzed by X-ray diffraction (XRD), scanning electron microscope (SEM), Raman spectrometer (RS), and X-ray photoelectron spectroscopy (XPS). Cyclic voltammograms gave information on the nature of electron transfer between nitrogen species and nickel foam electrode and revealed the potential dependence nature of ammonia oxidation over the potential window of +0.7 V to +0.85 V (vs. Hg/HgO). Batch controlled potential experiments using nickel foam as the working anode in a three-electrode system were conducted to study the oxidation of ammonia in solution containing 0.1 M of Na2SO4 electrolyte, at pH 11 and temperature of 25 °C. Based on the current efficiency and reaction kinetics, it was possible to establish the mechanism of selective ammonia conversion to gaseous nitrogen and nitrate.
中文翻译:

泡沫镍电极上的电催化氨氧化:Ni(OH)2(s) -NiOOH (s)纳米催化剂的作用
使用开孔结构的泡沫镍电极通过电化学形成Ni(OH)2进行氨的直接电催化氧化/ NiOOH纳米花在特定的超电势下在镍基质上形成(方钠石相)。通过X射线衍射(XRD),扫描电子显微镜(SEM),拉曼光谱仪(RS)和X射线光电子能谱(XPS)分析电极表面。循环伏安图提供了有关氮物种与泡沫镍电极之间电子转移性质的信息,并揭示了在+0.7 V至+0.85 V(vs。Hg / HgO)的电势窗口内氨氧化的电势依赖性。在三电极系统中使用泡沫镍作为工作阳极进行了批量控制电势实验,以研究含0.1 M Na 2 SO 4的溶液中氨的氧化电解液,pH为11,温度为25°C。基于电流效率和反应动力学,可以建立将氨选择性转化为气态氮和硝酸盐的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号