European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.ejmech.2018.01.026 Haydi Saher ElBordiny 1 , Mostafa Mahmoud El-Miligy 2 , Shaymaa Emam Kassab 1 , Hoda Daabees 1 , Waleed Ali Mohamed Ali 3 , Soad Abdelhamid Mohamed El-Hawash 2
|
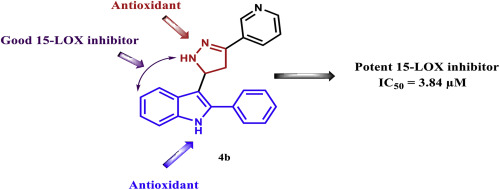
New candidates of 3-(4,5-dihydro-1H-pyrazol/isoxazol-5-yl)-2-phenyl-1H-indole derivatives (4–7) were designed combining the pyrazoline/isoxazoline heterocycles and 2-phenylindole to explore its potential as 15-lipoxygenase (15-LOX) inhibitors. The design of the new derivatives was based on utilizing the antioxidant properties of pyrazoline, 2-phenylindole and the good 15-LOX inhibition properties of indolylpyrazoline. The derivatives were synthesized adopting simple and laboratory friendly reaction conditions to give the target compounds in quantitative yields. The resulting indolylpyrazolines/isoxazolines were evaluated as antioxidants against 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO) and superoxide dismutase (SOD); indolylpyrazoline (4b) was the most potent antioxidant against SOD assay (IC50 = 1.78 μM) to be superior to ascorbic by 2 folds. Consistently, (4b) was the most potent inhibitor when tested against Soybean 15-LOX (IC50 = 3.84 μM) excelling quercetin as standard inhibitor by 1.8 folds. Some of the new derivatives were docked into the active binding site of human 15-LOX (PDB entry 4NRE) emphasizing the most potent derivative (4b) and the least potent one (4c). Docking solutions of compounds (4b), (4c), (5b) and (6c) revealed that (4b) was the only compound that got stabilized into the catalytic pocket of enzyme by π-cation interaction with the catalytic Fe+ and formation of one hydrogen bond with Ile 676 amino acid. Other derivatives including the least potent one variably got stabilized into the active binding pocket by π-cation interaction with the catalytic Fe+ but failed to form hydrogen bond with Ile 676. For the future optimization of the generated inhibitors, (i) antioxidant activity against SOD, (ii) the inhibitor stabilization by π-cation interaction with the catalytic Fe+3 and (iii) formation of hydrogen bond with Ile 676 should be regarded.
中文翻译:

新型3-(4,5-二氢-1H-吡唑/异恶唑-5-基)-2-苯基-1H-吲哚衍生物作为有效抗氧化剂和15-脂氧合酶抑制剂的设计、合成、生物学评价和对接研究
结合吡唑啉/异恶唑啉杂环和2-苯基吲哚,设计了新候选的3-(4,5-二氢-1H-吡唑/异恶唑-5-基)-2-苯基-1H-吲哚衍生物(4-7)探索其作为 15-脂氧合酶 (15-LOX) 抑制剂的潜力。新衍生物的设计基于吡唑啉、2-苯基吲哚的抗氧化特性和吲哚基吡唑啉良好的15-LOX抑制特性。采用简单且实验室友好的反应条件合成衍生物,以定量产率得到目标化合物。所得吲哚基吡唑啉/异恶唑啉作为抗 2,2-二苯基-1-三硝基苯肼 (DPPH)、一氧化氮 (NO) 和超氧化物歧化酶 (SOD) 的抗氧化剂进行了评估;吲哚吡唑啉(4b)是针对 SOD 测定最有效的抗氧化剂 (IC 50 = 1.78 μM),优于抗坏血酸 2 倍。一致地,当针对大豆 15-LOX (IC 50 = 3.84 μM) 进行测试时, (4b)是最有效的抑制剂,优于作为标准抑制剂的槲皮素 1.8 倍。一些新衍生物对接至人 15-LOX(PDB 条目 4NRE)的活性结合位点,强调最强的衍生物(4b)和最弱的衍生物(4c) 。化合物(4b) 、 (4c) 、 (5b)和(6c)的对接溶液表明, (4b)是唯一通过 π-阳离子与催化 Fe +相互作用并形成与Ile 676氨基酸有一个氢键。 其他衍生物,包括效力最弱的衍生物,通过 π-阳离子与催化 Fe +的相互作用,不同程度地稳定在活性结合袋中,但未能与 Ile 676 形成氢键。对于生成的抑制剂的未来优化,(i) 抗氧化活性SOD,(ii) 通过 π-阳离子与催化 Fe +3相互作用而稳定抑制剂,以及 (iii) 与 Ile 676 形成氢键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号