Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.bioorg.2018.01.012 Jessica B Pickens 1 , Logan G Mills 1 , Feng Wang 1 , Susanne Striegler 1
|
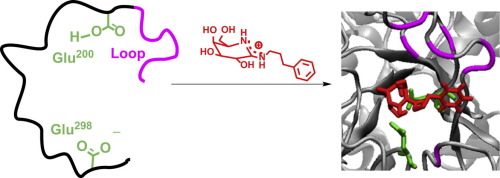
A spectroscopic examination of six galactonoamidines with inhibition constants and efficacy in the low nanomolar concentration range (Ki = 6–11 nM, IC50 = 12–36 nM) suggested only two of them as putative transition state analogs for the hydrolysis of β-galactosides by β-galactosidase (A. oryzae). A rationale for the experimental results was elaborated using docking and molecular dynamics studies. An analysis of the combined observations reveals several common factors of the compounds suggested as transition state analogs (TSAs): the putative TSAs have a similar orientation in the active site; show conserved positioning of the glycon; display a large number of H-bond interactions toward the catalytically active amino acid residues via their glycon; and exhibit hydrophobic interactions at the outer rim of the active site with small changes of the position and orientation of their respective aglycons.
中文翻译:

评估疏水性半乳糖脒作为酶促 β-半乳糖苷水解的过渡态类似物
对六种半乳糖脒的抑制常数和功效在低纳摩尔浓度范围(K i = 6–11 nM,IC 50 = 12–36 nM)进行的光谱检查表明,只有其中两种可以作为β-水解的推定过渡态类似物 -通过β-半乳糖苷酶 ( A. oryzae ) 产生半乳糖苷。使用对接和分子动力学研究详细阐述了实验结果的基本原理。对综合观察结果的分析揭示了建议作为过渡态类似物 (TSA) 的化合物的几个共同因素:假定的 TSA 在活性位点具有相似的方向;显示糖基的保守定位;通过其糖基对催化活性氨基酸残基表现出大量的氢键相互作用;并在活性位点的外缘表现出疏水相互作用,其各自的苷元的位置和方向发生微小变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号