当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anionic Triflyldiazomethane: Generation and Its Application for Synthesis of Pyrazole-3-triflones via [3 + 2] Cycloaddition Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.orglett.7b03664 Pulakesh Das 1 , Satoshi Gondo 1 , Etsuko Tokunaga 1 , Yuji Sumii 1 , Norio Shibata 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.orglett.7b03664 Pulakesh Das 1 , Satoshi Gondo 1 , Etsuko Tokunaga 1 , Yuji Sumii 1 , Norio Shibata 1, 2
Affiliation
|
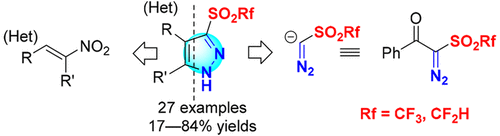
The synthesis of pyrazole triflones containing a triflyl group at the 3-position is disclosed. Treatment of 2-diazo-1-phenyl-2-((trifluoromethyl)sulfonyl)ethan-1-one with nitroalkenes under basic conditions gave pharmaceutically attractive pyrazole 3-triflones in good to high yields. The generation of anionic triflyldiazomethane species followed by the [3 + 2] cycloaddition reaction with nitroalkenes is proposed for this transformation. 3-(Difluoromethanesulfonyl)pyrazoles were also synthesized by using a previously unknown anionic (difluoromethanesulfonyl)diazomethane species under a similar strategy.
中文翻译:

阴离子三重氮唑甲烷:通过[3 + 2]环加成反应生成吡咯-3-三氟甲磺酸酯及其应用
公开了在3-位上含有三氟甲基的吡唑三氟酮的合成。在碱性条件下用硝基烯烃处理2-重氮-1-苯基-2-((三氟甲基)磺酰基)乙-1-酮,以良好或高收率得到了药学上有吸引力的吡唑3-三氟甲磺酸酯。对于该转化,提出了生成阴离子三重氮重氮甲烷物质,然后与硝基烯烃进行[3 + 2]环加成反应。还通过在类似策略下使用先前未知的阴离子(二氟甲磺酰基)重氮甲烷物质合成了3-(二氟甲磺酰基)吡唑。
更新日期:2018-01-10
中文翻译:

阴离子三重氮唑甲烷:通过[3 + 2]环加成反应生成吡咯-3-三氟甲磺酸酯及其应用
公开了在3-位上含有三氟甲基的吡唑三氟酮的合成。在碱性条件下用硝基烯烃处理2-重氮-1-苯基-2-((三氟甲基)磺酰基)乙-1-酮,以良好或高收率得到了药学上有吸引力的吡唑3-三氟甲磺酸酯。对于该转化,提出了生成阴离子三重氮重氮甲烷物质,然后与硝基烯烃进行[3 + 2]环加成反应。还通过在类似策略下使用先前未知的阴离子(二氟甲磺酰基)重氮甲烷物质合成了3-(二氟甲磺酰基)吡唑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号