当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Free-Energy Analysis of Peptide Binding in Lipid Membrane Using All-Atom Molecular Dynamics Simulation Combined with Theory of Solutions
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.jpcb.7b08241 Tomoko Mizuguchi 1, 2 , Nobuyuki Matubayasi 3, 4
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.jpcb.7b08241 Tomoko Mizuguchi 1, 2 , Nobuyuki Matubayasi 3, 4
Affiliation
|
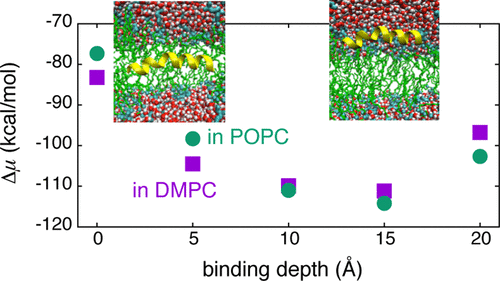
All-atom molecular dynamics (MD) simulations are performed to examine the stabilities of a variety of binding configurations of alamethicin, a 20-amino-acid amphipathic peptide, in the bilayers of 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) and 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC). The binding free energy of alamethicin is calculated through a combination of MD simulation and the energy-representation theory of solutions, and it is seen that the transmembrane configuration is stable in both membranes. A surface-bound state is also found to be stable due to the balance between the attractive and repulsive interactions of the peptide with lipid and water, and the key role of water is pointed out for the stability in the interfacial region. A difference between the POPC and DMPC systems is noted when the polar C-terminal domain is buried in the hydrophobic region of the membrane. In POPC, the peptide is unfavorably located with that configuration due to the loss of electrostatic interaction between the peptide and lipid.
中文翻译:

结合溶液理论的全原子分子动力学模拟脂质膜中肽结合的自由能分析
进行全原子分子动力学(MD)模拟以检查1-palmitoyl-2-oleoyl磷脂酰胆碱(POPC)和1的双层中alamethicin(一种20个氨基酸的两亲性肽)的各种结合构型的稳定性。 ,2-二肉豆蔻酰-甘油-3-磷脂酰胆碱(DMPC)。通过结合MD模拟和溶液的能量表示理论,计算了Alamicicin的结合自由能,可以看出两膜的跨膜构型都是稳定的。由于肽与脂质和水之间的吸引和排斥相互作用之间的平衡,还发现表面结合状态是稳定的,并且指出水的关键作用是界面区域的稳定性。当极性C末端结构域埋在膜的疏水区中时,会注意到POPC和DMPC系统之间的差异。在POPC中,由于肽和脂质之间的静电相互作用的损失,使得肽以该构型不利地定位。
更新日期:2018-01-10
中文翻译:

结合溶液理论的全原子分子动力学模拟脂质膜中肽结合的自由能分析
进行全原子分子动力学(MD)模拟以检查1-palmitoyl-2-oleoyl磷脂酰胆碱(POPC)和1的双层中alamethicin(一种20个氨基酸的两亲性肽)的各种结合构型的稳定性。 ,2-二肉豆蔻酰-甘油-3-磷脂酰胆碱(DMPC)。通过结合MD模拟和溶液的能量表示理论,计算了Alamicicin的结合自由能,可以看出两膜的跨膜构型都是稳定的。由于肽与脂质和水之间的吸引和排斥相互作用之间的平衡,还发现表面结合状态是稳定的,并且指出水的关键作用是界面区域的稳定性。当极性C末端结构域埋在膜的疏水区中时,会注意到POPC和DMPC系统之间的差异。在POPC中,由于肽和脂质之间的静电相互作用的损失,使得肽以该构型不利地定位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号