当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A highly efficient nucleophilic substitution reaction between R2P(O)H and triarylmethanols to synthesize phosphorus-substituted triarylmethanes†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7ob02970e Long Chen 1, 2, 3, 4 , Xin-Yue Fang 1, 2, 3, 4 , Yun-Xiang Zou 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7ob02970e Long Chen 1, 2, 3, 4 , Xin-Yue Fang 1, 2, 3, 4 , Yun-Xiang Zou 1, 2, 3, 4
Affiliation
|
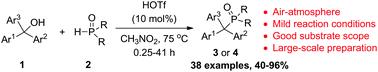
A highly efficient and general nucleophilic substitution reaction between dialkyl H-phosphonates or diarylphosphine oxides and triarylmethanols catalyzed by HOTf (trifluoromethanesulfonic acid) has been developed. It provides an atom-economical protocol for the synthesis of various symmetrical and unsymmetrical phosphorus-substituted triarylmethanes that constitute an emerging family of potent anticancer agents in rich diversity with 40 to 96% yields. The synthetic applicability of this protocol is demonstrated by gram-scale preparations.
中文翻译:

R 2 P(O)H与三芳基甲醇之间的高效亲核取代反应,可合成磷取代的三芳基甲烷†
已经开发了由HOTf(三氟甲磺酸)催化的H-膦酸二烷基酯或二芳基膦氧化物与三芳基甲醇之间的高效且通用的亲核取代反应。它为合成各种对称和不对称的磷取代的三芳基甲烷提供了一种原子经济的方法,这些化合物构成了新兴的强效抗癌剂家族,具有丰富的多样性,产率为40%至96%。该协议的综合适用性已通过克量级的制备证明。
更新日期:2018-01-10
中文翻译:

R 2 P(O)H与三芳基甲醇之间的高效亲核取代反应,可合成磷取代的三芳基甲烷†
已经开发了由HOTf(三氟甲磺酸)催化的H-膦酸二烷基酯或二芳基膦氧化物与三芳基甲醇之间的高效且通用的亲核取代反应。它为合成各种对称和不对称的磷取代的三芳基甲烷提供了一种原子经济的方法,这些化合物构成了新兴的强效抗癌剂家族,具有丰富的多样性,产率为40%至96%。该协议的综合适用性已通过克量级的制备证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号