当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aerogen bonds formed between AeOF2 (Ae = Kr, Xe) and diazines: comparisons between σ-hole and π-hole complexes†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7cp08048d Wiktor Zierkiewicz 1, 2, 3, 4 , Mariusz Michalczyk 1, 2, 3, 4 , Steve Scheiner 5, 6, 7, 8
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7cp08048d Wiktor Zierkiewicz 1, 2, 3, 4 , Mariusz Michalczyk 1, 2, 3, 4 , Steve Scheiner 5, 6, 7, 8
Affiliation
|
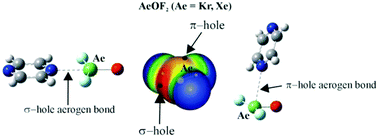
The interaction between KrOF2 or XeOF2 and the 1,2, 1,3, and 1,4 diazines is characterized chiefly by a Kr/Xe⋯N aerogen bond, as deduced from ab initio calculations. The most stable dimers take advantage of the σ-hole on the aerogen atom, wherein the two molecules lie in the same plane. The interaction is quite strong, as much as 18 kcal mol−1. A second class of dimer geometry utilizes the π-hole above the aerogen atom in an approximate perpendicular arrangement of the two monomers; these structures are not as strongly bound: 6–8 kcal mol−1. Both sorts of dimers contain auxiliary CH⋯F H-bonds which contribute to their stability, but even with their removal, the aerogen bond energy remains as high as 14 kcal mol−1. The nature and strength of each specific interaction is confirmed and quantified by AIM, NCI, NBO, and electron density shift patterns. There is not a great deal of sensitivity to the identity of either the aerogen atom or the position of the two N atoms in the diazine.
中文翻译:

AeOF 2(Ae = Kr,Xe)与二嗪之间形成的气键:σ-孔和π-孔配合物的比较†
KrOF之间的相互作用2或XeOF 2和1,2-,1,3-和1,4-二嗪是由氪/氙⋯Ñ键的Aerogen主要特征,如从推导出从头计算。最稳定的二聚体利用了气源原子上的σ孔,其中两个分子位于同一平面上。相互作用非常强,高达18 kcal mol -1。第二类二聚体几何结构是利用气孔原子上方的π孔以两种单体近似垂直的方式排列的。这些结构没有那么牢固的结合:6–8 kcal mol -1。两种类型的二聚体均包含有助于其稳定性的辅助CH⋯F H键,但即使将其除去,气原键能仍保持高达14 kcal mol -1。每种特定相互作用的性质和强度均通过AIM,NCI,NBO和电子密度转换模式进行了确认和量化。对气源原子的身份或二嗪中两个N原子的位置没有很大的敏感性。
更新日期:2018-01-10
中文翻译:

AeOF 2(Ae = Kr,Xe)与二嗪之间形成的气键:σ-孔和π-孔配合物的比较†
KrOF之间的相互作用2或XeOF 2和1,2-,1,3-和1,4-二嗪是由氪/氙⋯Ñ键的Aerogen主要特征,如从推导出从头计算。最稳定的二聚体利用了气源原子上的σ孔,其中两个分子位于同一平面上。相互作用非常强,高达18 kcal mol -1。第二类二聚体几何结构是利用气孔原子上方的π孔以两种单体近似垂直的方式排列的。这些结构没有那么牢固的结合:6–8 kcal mol -1。两种类型的二聚体均包含有助于其稳定性的辅助CH⋯F H键,但即使将其除去,气原键能仍保持高达14 kcal mol -1。每种特定相互作用的性质和强度均通过AIM,NCI,NBO和电子密度转换模式进行了确认和量化。对气源原子的身份或二嗪中两个N原子的位置没有很大的敏感性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号