Chemical Physics ( IF 2.0 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.chemphys.2018.01.004 Tao Yu , Ying-Zhe Liu , Wei-Peng Lai
|
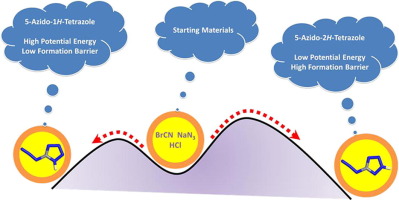
CHN7 and CN7- are meta-stable species. In order to study on the relationship between thermodynamic and kinetic stabilities, the potential energy surfaces of CHN7 and CN7- were scanned at the B3LYP/aug-cc-pVDZ level. After the analysis of potential energy surfaces, the optimum pathways were got to conclude the dissociation and formation mechanisms. The dissociation barriers of 5-azido-1H-tetrazole and 5-azido-2H-tetrazole are about 150 kJ mol-1. They are sufficient to keep the two azidotetrazoles stable. The reaction between cyanogen azide and azide anion cannot produce azidotetrazolate anion, but produce the linear CN7- with a lower barrier. The reaction between cyanogen azide and hydrazoic acid preferentially produce 5-azido-1H-tetrazole. The decyclization barriers of 1H-tetrazolo[1,5-d]tetrazole and tetrazolo[1,5-d]tetrazolate anion are 44.7 and 81.6 kJ mol-1, respectively. The deprotoned anion is more available than the neutral compound. Heptaazacubane and heptaazacubanide anion with cubic geometries are highly unstable.
中文翻译:

探索CHN的稳定性7和CN 7 - :在形成和分解机制的理论研究
CHN 7和CN 7 -是亚稳定的物种。为了研究关于热力学和动力学稳定性之间的关系,CHN的势能面7和CN 7 -在B3LYP / AUG-CC-pVDZ水平进行扫描。通过对势能面的分析,得出了最佳的途径来总结解离和形成机理。5-叠氮基1 H-四唑和5-叠氮基2 H-四唑的解离势垒约为150 kJ mol -1。它们足以保持两个叠氮四唑稳定。叠氮化氰和叠氮化物阴离子之间的反应不能产生叠氮四唑根阴离子,但会产生线性CN 7-具有较低的障碍。叠氮化氰和肼酸之间的反应优先产生5-叠氮基-1 H-四唑。1 H-四唑并[1,5-d]四唑和四唑并[1,5-d]四唑根阴离子的脱环势分别为44.7和81.6 kJ mol -1。去质子化的阴离子比中性化合物更容易获得。具有立方几何形状的庚七氮杂和庚二氮杂阴离子非常不稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号