Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.jinorgbio.2018.01.009 Igor Shamovsky 1 , Graham Belfield 1 , Richard Lewis 1 , Frank Narjes 1 , Lena Ripa 1 , Christian Tyrchan 1 , Lisa Öberg 1 , Peter Sjö 1
|
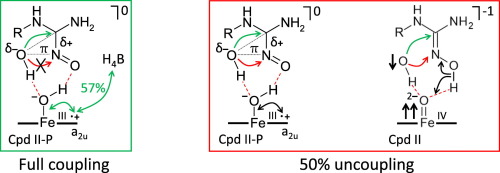
Nitric oxide (NO·) is a messenger molecule with diverse physiological roles including host defense, neurotransmission and vascular function. The synthesis of NO· from l-arginine is catalyzed by NO-synthases and occurs in two steps through the intermediary Nω-hydroxy-l-arginine (NHA). In both steps the P450-like reaction cycle is coupled with the redox cycle of the cofactor tetrahydrobiopterin (H4B). The mechanism of the second step is studied by Density Functional Theory calculations to ascertain the canonical sequence of proton and electron transfer (PT and ET) events. The proposed mechanism is controlled by the interplay of two electron donors, H4B and NHA. Consistent with experimental data, the catalytic cycle proceeds through the ferric-hydroperoxide complex (Cpd 0) and the following aqua-ferriheme resting state, and involves interim partial oxidation of H4B. The mechanism starts with formation of Cpd 0 from the ferrous-dioxy reactant complex by PT from the C-ring heme propionate coupled with hole transfer to H4B through the highest occupied π-orbital of NHA as a bridge. This enables PT from NHA+· to the proximal oxygen leading to the shallow ferriheme-H2O2 oxidant. Subsequent Fenton-like peroxide bond cleavage triggered by ET from the NHA-derived iminoxy-radical leads to the protonated Cpd II diradicaloid singlet stabilized by spin delocalization in H4B, and the closed-shell coordination complex of HO− with iminoxy-cation. The complex is converted to the transient C-adduct, which releases intended products upon PT to the ferriheme-HO− complex coupled with ET to the H4B+·. Deferred ET from the substrate or undue ET from/to the cofactor leads to side products.
中文翻译:

一氧化氮合酶反应第二步的理论研究:电子隧道效应阻止解偶联
一氧化氮(NO · )是一种具有多种生理作用的信使分子,包括宿主防御、神经传递和血管功能。由L -精氨酸合成 NO ·由 NO 合酶催化,并通过中间体N ω -羟基 - L -精氨酸 (NHA) 分两步进行。在这两个步骤中,类 P450 反应循环与辅因子四氢生物蝶呤 (H 4 B) 的氧化还原循环耦合。通过密度泛函理论计算研究第二步的机制,以确定质子和电子转移(PT 和 ET)事件的规范序列。所提出的机制由两个电子供体 H 4 B 和 NHA 的相互作用控制。与实验数据一致,催化循环通过铁-氢过氧化物络合物 (Cpd 0) 和随后的水-铁血红素静息状态进行,并涉及 H 4 B 的临时部分氧化。该机制从亚铁-氢过氧化物形成 Cpd 0 开始。二氧反应物络合物由来自C环血红素丙酸酯的PT与空穴通过NHA最高占据的π轨道作为桥转移至H 4 B。这使得PT从NHA + ·到近端氧,导致浅亚铁血红素-H 2 O 2氧化剂。随后由 ET 从 NHA 衍生的亚氨氧基自由基触发类芬顿过氧化物键裂解,产生通过 H 4 B 中自旋离域稳定的质子化 Cpd II 双自由基单线态,以及 HO -与亚氨氧基阳离子的闭壳配位络合物。 该复合物转化为瞬时 C-加合物,在 PT 形成铁血红素-HO −复合物并结合 ET 形成 H 4 B + ·后释放预期产物。来自底物的延迟 ET 或来自/至辅因子的过度 ET 会导致副产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号