Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study.
The Lancet ( IF 98.4 ) Pub Date : 2018-Apr-01 , DOI: 10.1016/s1473-3099(18)30002-1 John H Beigel 1 , Jocelyn Voell 2 , Parag Kumar 3 , Kanakatte Raviprakash 4 , Hua Wu 5 , Jin-An Jiao 5 , Eddie Sullivan 5 , Thomas Luke 6 , Richard T Davey 2
The Lancet ( IF 98.4 ) Pub Date : 2018-Apr-01 , DOI: 10.1016/s1473-3099(18)30002-1 John H Beigel 1 , Jocelyn Voell 2 , Parag Kumar 3 , Kanakatte Raviprakash 4 , Hua Wu 5 , Jin-An Jiao 5 , Eddie Sullivan 5 , Thomas Luke 6 , Richard T Davey 2
Affiliation
|
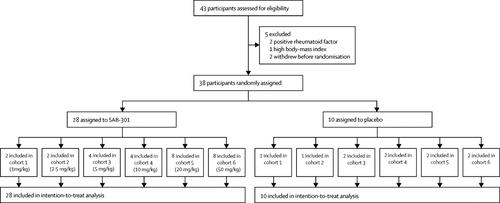
Middle East respiratory syndrome (MERS) is a severe respiratory illness with an overall mortality of 35%. There is no licensed or proven treatment. Passive immunotherapy approaches are being developed to prevent and treat several human medical conditions where alternative therapeutic options are absent. We report the safety of a fully human polyclonal IgG antibody (SAB-301) produced from the hyperimmune plasma of transchromosomic cattle immunised with a MERS coronavirus vaccine.
中文翻译:

由转染色体牛产生的新型多克隆人抗 MERS 冠状病毒抗体的安全性和耐受性:一项 1 期随机、双盲、单剂量递增研究。
中东呼吸综合征 (MERS) 是一种严重的呼吸系统疾病,总死亡率为 35%。没有获得许可或经过验证的治疗方法。被动免疫疗法正在开发中,以预防和治疗几种缺乏替代治疗选择的人类疾病。我们报告了一种全人多克隆 IgG 抗体 (SAB-301) 的安全性,该抗体是由接种 MERS 冠状病毒疫苗的转染色体牛的超免疫血浆产生的。
更新日期:2018-03-22
中文翻译:

由转染色体牛产生的新型多克隆人抗 MERS 冠状病毒抗体的安全性和耐受性:一项 1 期随机、双盲、单剂量递增研究。
中东呼吸综合征 (MERS) 是一种严重的呼吸系统疾病,总死亡率为 35%。没有获得许可或经过验证的治疗方法。被动免疫疗法正在开发中,以预防和治疗几种缺乏替代治疗选择的人类疾病。我们报告了一种全人多克隆 IgG 抗体 (SAB-301) 的安全性,该抗体是由接种 MERS 冠状病毒疫苗的转染色体牛的超免疫血浆产生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号