Electrochimica Acta ( IF 5.5 ) Pub Date : 2018-01-09 , DOI: 10.1016/j.electacta.2018.01.052 Salvador Cotillas , Javier Llanos , Pablo Cañizares , Davide Clematis , Giacomo Cerisola , Manuel A. Rodrigo , Marco Panizza
|
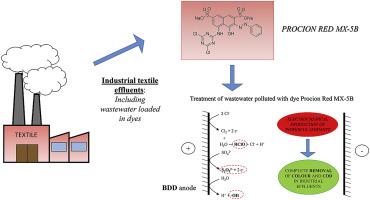
In this work, the removal of Procion Red MX-5B dye by electrochemical oxidation with boron doped diamond (BDD) anodes was investigated. The impact of current density, flow rate, initial pH, and supporting electrolyte was evaluated on dye and organic matter removal. Furthermore, the use of dimensionally stable anodes (DSA) was tested to evaluate process performance. Results show that after 240 min, it is possible to achieve full dye and COD (Chemical Oxygen Demand) removal, regardless of applied current density. This is due to the generation of powerful oxidants – i.e. hydroxyl radicals and peroxodisulfate–, which attack the organic matter in the wastewater, promoting its complete degradation. However, process efficiency increases when using lower current densities (10 mA cm−2): electric charges of about 5 Ah dm−3 are sufficient to fully remove both dye and COD, while charges higher than 15 Ah dm−3 are required when working at higher current densities (>30 mA cm−2). This fact is related to the production of large amounts of hydroxyl radicals, which are wasted in other reactions at higher current densities. On the other hand, higher flow rates (300 dm3 h−1) promote Procion Red MX-5B and organic matter degradation, due to improved mass transfer within the system. Regarding the impact of initial pH on dye removal, no significant differences were observed. Conversely, COD is clearly affected by this parameter: it is only possible to fully remove the organic matter when working at natural pH.
Finally, with DSA anodes, higher dye removal efficiencies are attained than with BDD electrodes, when 100 mg dm−3 chlorides are added to the supporting electrolyte. Likewise, higher chloride concentration (100–1000 mg dm−3) was observed to enhance process efficiency when using DSA as anode material. However, during electrolysis with both BDD and DSA, chloride ions in the supporting electrolyte promote the production of intermediate organochlorinated compounds. Therefore, under these conditions, no full organic matter removal can be achieved, regardless of the anode material employed.
中文翻译:

导电金刚石电化学氧化去除废水中的Procion Red MX-5B染料
在这项工作中,研究了用硼掺杂金刚石(BDD)阳极进行电化学氧化去除Procion Red MX-5B染料的方法。评估了电流密度,流速,初始pH和支持电解质对染料和有机物去除的影响。此外,还测试了尺寸稳定阳极(DSA)的使用,以评估工艺性能。结果表明,在240分钟之后,无论施加何种电流密度,都可以完全去除染料和COD(化学需氧量)。这是由于产生了强大的氧化剂,即羟基自由基和过二硫酸盐,它们攻击废水中的有机物,促进了废水的完全降解。但是,使用较低的电流密度(10 mA cm -2时,过程效率会提高):约5 Ah dm -3的电荷足以完全去除染料和COD,而在更高电流密度(> 30 mA cm -2)下工作时,则需要高于15 Ah dm -3的电荷。这个事实与大量羟基自由基的产生有关,这些羟基自由基在其他反应中以较高的电流密度被浪费掉了。另一方面,更高的流速(300 dm 3 h -1)由于改善了系统内的传质能力,导致Procion Red MX-5B和有机物降解。关于初始pH对染料去除的影响,未观察到显着差异。相反,COD显然受此参数影响:只有在自然pH下工作时,才可能完全除去有机物。
最终,当将100 mg dm -3氯化物添加到支持电解质中时,使用DSA阳极,可以获得比BDD电极更高的染料去除效率。同样,当使用DSA作为阳极材料时,观察到较高的氯化物浓度(100–1000 mg dm -3)可提高工艺效率。但是,在用BDD和DSA进行电解期间,支持电解质中的氯离子会促进中间体有机氯化合物的生成。因此,在这些条件下,无论采用何种阳极材料,都无法完全去除有机物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号