Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.apcata.2018.01.010 Xiaoli Zheng , Sibei Guo , Ling Guo
|
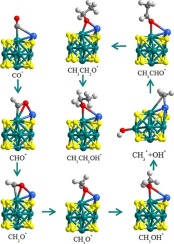
Conversion of carbon monoxide (CO) into ethanol has attracted significant interest due to ethanol can be used as alternative energy sources to satisfy the rising global energy demands and to solve the serious environmental problems, although it suffers from slow kinetics and low selectivity. Metal single-atom catalyst of Ni single atom supported on Mo6S8 (Ni-SA/Mo6S8) catalyst was developed for the exclusive conversion of CO into ethanol. Herein, ethanol synthesis on Ni-SA/Mo6S8 was systematically investigated using density functional theory (DFT) and microkinetic modeling. The adsorption situations and the reaction routes for ethanol reactions on Ni-SA/Mo6S8 were clarified. Interestingly, the selectivity to ethanol is controlled by CH3 formation and CC bond formation between CH3 species and CHO. Our results indicated that Ni-SA/Mo6S8 increases the stability of the reaction intermediates, and CH3 is the most favorable formed by CH3OH dissociation. Then CHO insertion into CH3 to form CH3CHO* is spontaneous, which suggested that the formation of ethanol is the most favorable on the Ni-SA/Mo6S8 catalyst. The BEP relationships of five different types of bonds in ethanol synthesis identified C
O bond scission does not readily occur compared to the other four types of bonds, the formation of C
H, O
H, and C
C bonds are easy to occur. Microkinetic modeling also confirmed Ni-SA/Mo6S8 shows extremely activity and selectivity for ethanol formation. We hope our study can provide the basis to understand and develop single-atom catalysis for ethanol synthesis.
中文翻译:

Mo 6 S 8载体上负载的单Ni原子催化合成乙醇。
一氧化碳(CO)转化为乙醇引起了广泛的兴趣,因为乙醇可以用作替代能源,以满足不断增长的全球能源需求并解决严重的环境问题,尽管乙醇动力学缓慢且选择性低。开发了负载在Mo 6 S 8(Ni-SA / Mo 6 S 8)催化剂上的Ni单原子金属单原子催化剂,用于将CO独家转化为乙醇。本文中,使用密度泛函理论(DFT)和微动力学模型系统地研究了Ni-SA / Mo 6 S 8上的乙醇合成。Ni-SA / Mo 6上乙醇反应的吸附情况和反应路线澄清了S 8。有趣的是,乙醇的选择性是由控制CH 3形成和C CH C之间键的形成3种和CHO。我们的结果表明,Ni-SA / Mo 6 S 8增加了反应中间体的稳定性,而CH 3是通过CH 3 OH分解最有利的形式。然后自发地将CHO插入CH 3形成CH 3 CHO *,这表明乙醇的形成对Ni-SA / Mo 6 S 8最为有利。催化剂。
与其他四种类型的键相比,乙醇合成中五种不同类型的键的BEP关系确定了不容易发生C O键断裂,而C
H,OH
和C
C键的形成则容易发生。微动力学模型还证实了Ni-SA / Mo 6 S 8对乙醇形成具有极高的活性和选择性。我们希望我们的研究可以为理解和发展单原子催化乙醇合成提供基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号