Tetrahedron ( IF 2.1 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.tet.2018.01.009 Liza Saher , Malika Makhloufi-Chebli , Leila Dermeche , Samia Dermeche , Baya Boutemeur-Khedis , Cherifa Rabia , Maamar Hamdi , Artur M.S. Silva
|
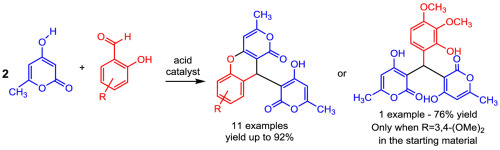
A series of novel 10-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-3-methyl-1H,10H-pyrano[4,3-b]chromen-1-ones were synthesized by a pseudo-three-component reaction of 4-hydroxy-6-methyl-2-oxo-2H-pyran-2-one (TAL) with 2-hydroxyarylaldehydes using different acids as catalysts and solvents. The approach relies on a regioselective cascade reaction involving two molar equiv of the TAL iteratively acting as active methylene in a Knoevenagel condensation and in a Michael addition. The antioxidant activity of the synthesized compounds were determined using the DPPH scavenging assay, being the results dependent on the nature and number of chromone substituents. The compound bearing an ortho-dihydroxy (catechol) moiety showed excellent activity at lower concentrations, while derivatives bearing alkoxy groups as substituents present pro-oxidant activity.
中文翻译:

来自a的10-(4-羟基-6-甲基-2-氧代-2- H-吡喃-3-基)-3-甲基-1 H,10 H-吡喃[4,3 - b ] chromen -1- one假多组分反应及其抗氧化活性评估
一系列新颖的10-(4-羟基-6-甲基-2-氧代-2 H-吡喃-3-基)-3-甲基-1 H,10 H-吡喃[4,3- b ] chromen -1通过使用不同的酸作为催化剂和溶剂,通过4-羟基-6-甲基-2-氧-2- H -2吡喃-2-酮(TAL)与2-羟基芳基醛的拟三组分反应合成α-酮。该方法依赖于区域选择性级联反应,该反应涉及两个摩尔当量的TAL在Knoevenagel缩合反应和Michael加成反应中反复充当活性亚甲基。使用DPPH清除测定法测定合成的化合物的抗氧化活性,其结果取决于色酮取代基的性质和数量。邻位化合物-二羟基(邻苯二酚)部分在较低浓度下显示出优异的活性,而带有烷氧基作为取代基的衍生物则表现出前氧化活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号