Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simultaneous Binding of Folic Acid and Methotrexate to Human Serum Albumin: Insights into the Structural Changes of Protein and the Location and Competitive Displacement of Drugs
ACS Omega ( IF 3.7 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acsomega.7b01437 Sudipta Panja 1 , Deb Kumar Khatua 1 , Mintu Halder 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acsomega.7b01437 Sudipta Panja 1 , Deb Kumar Khatua 1 , Mintu Halder 1
Affiliation
|
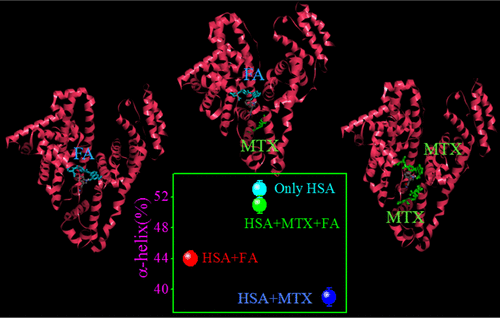
Protein structure can be flexible to adopt multiple conformations to house small molecules. In this paper, we have attempted to experimentally figure out how the structure of a transport protein steer the drug–drug competition (DDC) by maintaining the equilibrium distribution of the bound and unbound fractions of drugs. This understanding is an important facet in biophysical and medicinal chemistry to ascertain the effectiveness of drugs. It is important to note that majority of studies involving small-molecule–transport protein interaction aimed at characterizing the binding process, and because these proteins can interact with thousands of molecules, there are hundreds of reports on such interactions. This ultimately led to an impression among the readers that any studies involving serum albumin may not lead to any new findings except for traditional binding explorations. However, in the present paper, we would like to draw the attention of the readers that our findings are very surprising, new, and important, involving the phenomenon of ligand–protein interaction. Here, we have studied two structurally similar drugs methotrexate (MTX) and folic acid (FA), which attempt to bind the primary binding site (subdomain IIA), one at a time, of human serum albumin. Details of binding analyses reveal that when both of the drugs are present, the single-site binding mode of FA prefers to occupy the primary binding site and hence pushes the primary-site-bound MTX to another location (subdomain IIIA), which is the second binding site of MTX. The structural analysis indicates that DDC has occurred in a cooperative fashion so that the loss of the protein secondary structure is minimum when both drugs are bound to the protein, which means that in the case of duo-drug binding, the conventional interaction between the drug and the protein is altered to undergo restoration of the protein structure. This can indeed regulate the DDC by modifying the bound and unbound fractions of MTX in plasma. The present study emphasizes that in vitro structural characterizations of the transport protein provide important information to improve the molecular-level understanding of DDC for further therapeutic applications with combination drug.
中文翻译:

叶酸和甲氨蝶呤与人血清白蛋白的同时结合:深入了解蛋白质的结构变化以及药物的位置和竞争性置换
蛋白质结构可以灵活地采用多种构象来容纳小分子。在本文中,我们试图通过维持药物的结合部分和未结合部分的平衡分布,尝试通过实验弄清转运蛋白的结构如何引导药物竞争。这种认识是确定药物有效性的生物物理和药物化学的重要方面。重要的是要注意,涉及小分子-转运蛋白相互作用的大多数研究旨在表征结合过程,并且由于这些蛋白可以与数千个分子相互作用,因此有数百种关于这种相互作用的报道。这最终在读者中引起了一种印象,即与血清白蛋白有关的任何研究都可能导致除传统结合探索之外的任何新发现。但是,在本文中,我们希望引起读者的注意,我们的发现非常令人惊讶,新颖且重要,涉及配体与蛋白质相互作用的现象。在这里,我们研究了两种结构相似的药物甲氨蝶呤(MTX)和叶酸(FA),它们试图结合人血清白蛋白的主要结合位点(亚结构域IIA),一次结合一个。结合分析的详细信息显示,当同时存在两种药物时,FA的单位点结合模式更倾向于占据主要结合位点,因此将结合主要位点的MTX推到另一个位置(子域IIIA),即MTX的第二个结合位点。结构分析表明,DDC以协作方式发生,因此当两种药物都结合到蛋白质时,蛋白质二级结构的损失最小,这意味着在二重药物结合的情况下,药物之间的常规相互作用蛋白质被改变以恢复其蛋白质结构。实际上,这可以通过修饰血浆中MTX的结合部分和未结合部分来调节DDC。本研究强调转运蛋白的体外结构表征提供了重要的信息,以提高对DDC的分子水平的了解,以进一步用于联合药物的治疗应用。
更新日期:2018-01-09
中文翻译:

叶酸和甲氨蝶呤与人血清白蛋白的同时结合:深入了解蛋白质的结构变化以及药物的位置和竞争性置换
蛋白质结构可以灵活地采用多种构象来容纳小分子。在本文中,我们试图通过维持药物的结合部分和未结合部分的平衡分布,尝试通过实验弄清转运蛋白的结构如何引导药物竞争。这种认识是确定药物有效性的生物物理和药物化学的重要方面。重要的是要注意,涉及小分子-转运蛋白相互作用的大多数研究旨在表征结合过程,并且由于这些蛋白可以与数千个分子相互作用,因此有数百种关于这种相互作用的报道。这最终在读者中引起了一种印象,即与血清白蛋白有关的任何研究都可能导致除传统结合探索之外的任何新发现。但是,在本文中,我们希望引起读者的注意,我们的发现非常令人惊讶,新颖且重要,涉及配体与蛋白质相互作用的现象。在这里,我们研究了两种结构相似的药物甲氨蝶呤(MTX)和叶酸(FA),它们试图结合人血清白蛋白的主要结合位点(亚结构域IIA),一次结合一个。结合分析的详细信息显示,当同时存在两种药物时,FA的单位点结合模式更倾向于占据主要结合位点,因此将结合主要位点的MTX推到另一个位置(子域IIIA),即MTX的第二个结合位点。结构分析表明,DDC以协作方式发生,因此当两种药物都结合到蛋白质时,蛋白质二级结构的损失最小,这意味着在二重药物结合的情况下,药物之间的常规相互作用蛋白质被改变以恢复其蛋白质结构。实际上,这可以通过修饰血浆中MTX的结合部分和未结合部分来调节DDC。本研究强调转运蛋白的体外结构表征提供了重要的信息,以提高对DDC的分子水平的了解,以进一步用于联合药物的治疗应用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号