当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and Diastereoselective Access to Fused Isoxazolidines via Ru(II)-Catalyzed C–H Activation of Nitrones and Coupling with Perfluoroalkylolefins
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.orglett.7b03775 Yunyun Li 1, 2 , Fang Xie 1 , Yuan Liu 1 , Xifa Yang 1, 2 , Xingwei Li 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.orglett.7b03775 Yunyun Li 1, 2 , Fang Xie 1 , Yuan Liu 1 , Xifa Yang 1, 2 , Xingwei Li 1
Affiliation
|
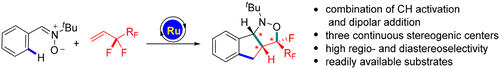
The synthsis of fluorinated isoxazolidines in bicyclic settings has been realized via Ru(II)-catalyzed C–H activation of aryl nitrones with perfluoroalkyl-substituted olefins as the coupling partner. The reaction proceeded via initial chelation-assisted C(aryl)–H allylation followed by regio- and diastereoselective intramolecular dipolar addition between the nitrone directing group and the olefin unit.
中文翻译:

通过Ru(II)催化硝基的CH活化并与全氟烷基烯烃偶联,对融合的异恶唑烷进行区域和非对映选择性反应
氟化异恶唑烷在双环环境中的合成已通过Ru(II)催化的全氟烷基取代的烯烃作为偶联伙伴的芳基硝酮的C–H活化。该反应通过初始螯合辅助的C(芳基)-H烯丙基化反应进行,然后在硝基导向基团和烯烃单元之间进行区域和非对映选择性分子内偶极加成。
更新日期:2018-01-09
中文翻译:

通过Ru(II)催化硝基的CH活化并与全氟烷基烯烃偶联,对融合的异恶唑烷进行区域和非对映选择性反应
氟化异恶唑烷在双环环境中的合成已通过Ru(II)催化的全氟烷基取代的烯烃作为偶联伙伴的芳基硝酮的C–H活化。该反应通过初始螯合辅助的C(芳基)-H烯丙基化反应进行,然后在硝基导向基团和烯烃单元之间进行区域和非对映选择性分子内偶极加成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号