当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermal and photocatalytic oxidation of organic substrates by dioxygen with water as an electron source
Green Chemistry ( IF 9.8 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7gc03387g Shunichi Fukuzumi 1, 2, 3, 4, 5 , Yong-Min Lee 1, 2, 3, 4, 6 , Jieun Jung 1, 2, 3, 4, 7 , Wonwoo Nam 1, 2, 3, 4, 8
Green Chemistry ( IF 9.8 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7gc03387g Shunichi Fukuzumi 1, 2, 3, 4, 5 , Yong-Min Lee 1, 2, 3, 4, 6 , Jieun Jung 1, 2, 3, 4, 7 , Wonwoo Nam 1, 2, 3, 4, 8
Affiliation
|
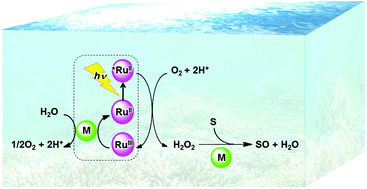
The oxidation of organic substrates is of fundamental importance in nature and includes key transformations in organic synthesis and industry. Dioxygen (O2) is potentially the greenest oxidant because O2 is available in air and its reduction product is water, which is environmentally the most benign substance. However, the direct thermal oxidation of organic substrates with O2 is spin-forbidden because the spin state of ground-state O2 is triplet and the spin state of most organic substrates is singlet. Transition metal complexes can react with triplet O2 in the presence of an electron source to produce metal–oxygen intermediates, such as metal-oxo and metal-hydroperoxo complexes, that can oxidize substrates under mild conditions. Various thermal and photocatalytic mechanisms of the oxidation of organic substrates by O2 with transition metal complexes have been critically discussed by focusing on how metal–oxygen intermediates are produced in the presence of an electron source and then react with organic substrates. Metal–oxygen intermediates are more easily produced by the reactions of metal complexes with hydrogen peroxide (H2O2) and act as active catalysts for the oxidation of substrates by H2O2. The photocatalytic oxidation of water by O2 to produce H2O2 has been combined with the catalytic oxidation of organic substrates by H2O2 to achieve the photocatalytic oxidation of organic substrates by O2. The photocatalytic oxidation of organic substrates by O2 with the use of water as an electron source has also been made possible by the use of inorganic and organic photocatalysts, which can be combined with transition metal complexes as catalysts for thermal reaction steps.
中文翻译:

以水为电子源的双氧热和光催化氧化有机底物
有机底物的氧化本质上具有根本的重要性,包括有机合成和工业中的关键转变。氧气(O 2)可能是最绿色的氧化剂,因为空气中有O 2,其还原产物是水,这是环境上最有益的物质。然而,由于基态O 2的自旋态为三重态且大多数有机基板的自旋态为单重态,因此禁止使用O 2对有机基板进行直接热氧化。过渡金属络合物可以与三重态O 2反应在电子源的存在下产生金属-氧中间体,例如金属-氧代和金属-氢过氧配合物,它们可以在温和的条件下氧化底物。通过重点讨论在电子源存在下如何生成金属-氧中间体,然后使其与有机底物发生反应的方法,对用O 2与过渡金属配合物氧化有机底物的各种热催化机理和光催化机理进行了严格的讨论。金属-氧中间体更容易被金属配合物与过氧化氢(H的反应中产生的2 ö 2),并且作为底物的氧化活性的催化剂用H 2 ö 2。O 2对水的光催化氧化生成H 2 ö 2已结合由H有机底物的催化氧化2 ö 2被O,实现有机基质的光催化氧化2。通过使用无机和有机光催化剂,还可以使用水作为电子源通过O 2对有机底物进行光催化氧化,所述无机和有机光催化剂可以与过渡金属配合物结合用作热反应步骤的催化剂。
更新日期:2018-03-06
中文翻译:

以水为电子源的双氧热和光催化氧化有机底物
有机底物的氧化本质上具有根本的重要性,包括有机合成和工业中的关键转变。氧气(O 2)可能是最绿色的氧化剂,因为空气中有O 2,其还原产物是水,这是环境上最有益的物质。然而,由于基态O 2的自旋态为三重态且大多数有机基板的自旋态为单重态,因此禁止使用O 2对有机基板进行直接热氧化。过渡金属络合物可以与三重态O 2反应在电子源的存在下产生金属-氧中间体,例如金属-氧代和金属-氢过氧配合物,它们可以在温和的条件下氧化底物。通过重点讨论在电子源存在下如何生成金属-氧中间体,然后使其与有机底物发生反应的方法,对用O 2与过渡金属配合物氧化有机底物的各种热催化机理和光催化机理进行了严格的讨论。金属-氧中间体更容易被金属配合物与过氧化氢(H的反应中产生的2 ö 2),并且作为底物的氧化活性的催化剂用H 2 ö 2。O 2对水的光催化氧化生成H 2 ö 2已结合由H有机底物的催化氧化2 ö 2被O,实现有机基质的光催化氧化2。通过使用无机和有机光催化剂,还可以使用水作为电子源通过O 2对有机底物进行光催化氧化,所述无机和有机光催化剂可以与过渡金属配合物结合用作热反应步骤的催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号