当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diverse bimetallic mechanisms emerging from transition metal Lewis acid/base pairs: development of co-catalysis with metal carbenes and metal carbonyl anions
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7cc09675e Neal P. Mankad 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7cc09675e Neal P. Mankad 1, 2, 3, 4
Affiliation
|
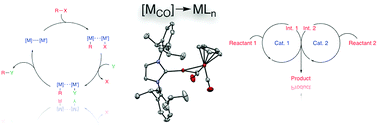
The rational development of catalytic reactions involving cooperative behavior between two catalytic reactive sites represents a frontier area of research from which novel reactivity and selectivity patterns emerge. Within this context, this Feature highlights the development of a cooperative system involving transition metal Lewis acid/base pairs. Bimetallic systems consisting of copper carbene Lewis acids and metal carbonyl anion Lewis bases, (NHC)Cu–[MCO], are easily synthesized from readily available organometallic building blocks (NHC = N-heterocyclic carbene; [MCO]− = metal carbonyl anion, e.g. [FeCp(CO)2]−, [Mn(CO)5]−, etc.). Stoichiometric reactivity studies indicate that the dative Cu←M bonds in these systems are labile towards heterolysis under mild conditions, thus providing in situ access both to polar metal–metal bonds and to “frustrated” transition metal Lewis acid/base pairs as dictated by reaction conditions. Catalytic transformations ranging from C–C and C–B coupling reactions to hydrogenation and other reductions have been developed from both manifolds: bimetallic catalysis involving (a) binuclear intermediates engaging in cooperative bond activation and formation, and (b) orthogonal mononuclear intermediates that operate in either tandem or co-dependent manners. Preliminary indications point to the future emergence of novel reactivity and selectivity patterns as these new motifs undergo continued development, and additionally demonstrate that the relative matching of two reactive sites provides a method for controlling catalytic behavior. Collectively, these results highlight the fundamental importance of exploring unconventional catalytic paradigms.
中文翻译:

过渡金属路易斯酸/碱对中出现的多种双金属机理:与金属卡宾和羰基金属阴离子共催化的发展
涉及两个催化反应位点之间协同行为的催化反应的合理发展代表了一个前沿的研究领域,由此产生了新的反应性和选择性模式。在此背景下,此功能着重介绍了涉及过渡金属路易斯酸/碱对的协同系统的开发。由卡宾铜路易斯酸和羰基金属阴离子路易斯碱(NHC)Cu– [M CO ]组成的双金属体系很容易从容易获得的有机金属结构单元(NHC = N-杂环卡宾; [M CO ] - =羰基金属)合成阴离子,例如[FeCp(CO)2 ] -,[Mn(CO)5 ] -,等)。化学计量的反应性的研究表明,在这些系统中的配价的Cu←中号键是朝向温和的条件下heterolysis不稳定的,因此提供了在原位如反应条件所指示,可同时访问极性金属-金属键和“受阻的”过渡金属路易斯酸/碱对。两种歧管均已开发出从C–C和C–B偶联反应到加氢和其他还原反应的催化转化:双金属催化涉及(a)参与协作键活化和形成的双核中间体,以及(b)运行的正交单核中间体以串联或相互依赖的方式。初步迹象表明,随着这些新基序的不断发展,新的反应性和选择性模式将在未来出现,并进一步证明两个反应位的相对匹配为控制催化行为提供了一种方法。总的来说,
更新日期:2018-02-02
中文翻译:

过渡金属路易斯酸/碱对中出现的多种双金属机理:与金属卡宾和羰基金属阴离子共催化的发展
涉及两个催化反应位点之间协同行为的催化反应的合理发展代表了一个前沿的研究领域,由此产生了新的反应性和选择性模式。在此背景下,此功能着重介绍了涉及过渡金属路易斯酸/碱对的协同系统的开发。由卡宾铜路易斯酸和羰基金属阴离子路易斯碱(NHC)Cu– [M CO ]组成的双金属体系很容易从容易获得的有机金属结构单元(NHC = N-杂环卡宾; [M CO ] - =羰基金属)合成阴离子,例如[FeCp(CO)2 ] -,[Mn(CO)5 ] -,等)。化学计量的反应性的研究表明,在这些系统中的配价的Cu←中号键是朝向温和的条件下heterolysis不稳定的,因此提供了在原位如反应条件所指示,可同时访问极性金属-金属键和“受阻的”过渡金属路易斯酸/碱对。两种歧管均已开发出从C–C和C–B偶联反应到加氢和其他还原反应的催化转化:双金属催化涉及(a)参与协作键活化和形成的双核中间体,以及(b)运行的正交单核中间体以串联或相互依赖的方式。初步迹象表明,随着这些新基序的不断发展,新的反应性和选择性模式将在未来出现,并进一步证明两个反应位的相对匹配为控制催化行为提供了一种方法。总的来说,











































 京公网安备 11010802027423号
京公网安备 11010802027423号