当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorescence spectroscopy reveals N-terminal order in fibrillar forms of α-synuclein
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7cc08601f Conor M Haney 1 , E James Petersson
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7cc08601f Conor M Haney 1 , E James Petersson
Affiliation
|
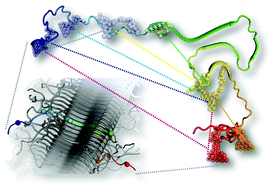
The neuronal protein α-synuclein (αS) plays a key role in Parkinson's disease, forming inclusions termed Lewy bodies and Lewy neurites. Recent improvements in cryo-electron diffraction and solid state NMR (ssNMR) have led to the elucidation of the structures of peptides derived from the αS fibril core and full-length human αS in fibrils. Despite the valuable insight offered by these methods, there are still several questions about the structures’ relevance to pathological aggregates. Herein, we present fluorescence data collected in vitro under the conditions which fibrils are typically assembled. Our data suggest that, in solution, fibrils are largely structured as observed by ssNMR. However, we observe significant disparities in the αS N-terminus as compared to ssNMR data, which provide insight on its important role in αS aggregation and fibril structure.
中文翻译:

荧光光谱揭示了 α-突触核蛋白纤维状形式的 N 末端顺序
神经元蛋白 α-突触核蛋白 (αS) 在帕金森病中发挥着关键作用,形成称为路易体和路易神经突的包涵体。冷冻电子衍射和固态核磁共振 (ssNMR) 的最新进展阐明了源自 αS 原纤维核心和原纤维中全长人 αS 的肽的结构。尽管这些方法提供了有价值的见解,但关于这些结构与病理聚集体的相关性仍然存在一些问题。在此,我们展示了在原纤维通常组装的条件下体外收集的荧光数据。我们的数据表明,在溶液中,原纤维很大程度上是通过单链核磁共振观察到的结构。然而,与 ssNMR 数据相比,我们观察到 αS N 末端存在显着差异,这为了解其在 αS 聚集和原纤维结构中的重要作用提供了见解。
更新日期:2018-01-18
中文翻译:

荧光光谱揭示了 α-突触核蛋白纤维状形式的 N 末端顺序
神经元蛋白 α-突触核蛋白 (αS) 在帕金森病中发挥着关键作用,形成称为路易体和路易神经突的包涵体。冷冻电子衍射和固态核磁共振 (ssNMR) 的最新进展阐明了源自 αS 原纤维核心和原纤维中全长人 αS 的肽的结构。尽管这些方法提供了有价值的见解,但关于这些结构与病理聚集体的相关性仍然存在一些问题。在此,我们展示了在原纤维通常组装的条件下体外收集的荧光数据。我们的数据表明,在溶液中,原纤维很大程度上是通过单链核磁共振观察到的结构。然而,与 ssNMR 数据相比,我们观察到 αS N 末端存在显着差异,这为了解其在 αS 聚集和原纤维结构中的重要作用提供了见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号