当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Palladium‐Based Electrochemical Sensor for Quantitative Detection of Formaldehyde
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-01-09 , DOI: 10.1002/slct.201702019 Ernest O. Nachaki 1 , Peter M. Ndangili 2 , Noah M. Naumih 3 , Eric Masika 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-01-09 , DOI: 10.1002/slct.201702019 Ernest O. Nachaki 1 , Peter M. Ndangili 2 , Noah M. Naumih 3 , Eric Masika 1
Affiliation
|
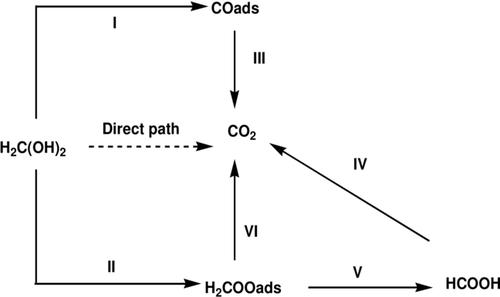
Formaldehyde is a small organic molecule that has a wide range of uses in society despite its toxicity. Formaldehyde is classified as a “known carcinogen” by International Agency for Research on Cancer (IARC). Formaldehyde electrooxidation has become a subject of major interest in the recent past due to its potential application in fuel cell technology and the need for its detection at trace levels because of its toxicity. Many studies have been conducted on formaldehyde electrooxidation, most of which suffer electrode passivation as a result of adsorbed intermediates such as carbon monoxide adsorbed (COads) and formic acid adsorbed (H2COOads) formed from electrooxidation of formaldehyde. In this study a Nickel Palladium nanoparticles modified glassy carbon electrode (Ni−Pd/GCE) was fabricated for electrooxidation of formaldehyde. Palladium nanoparticles were electrochemically deposited onto a bare Glassy Carbon Electrode (GCE) from 2 mM PdCl2 in 0.1 M H2SO4 supporting electrolyte, at a controlled potential of −0.14 V for 240 seconds. The Nickel nanoparticles were electrochemically deposited onto the PdGCE from 0.5 M NiSO4 in 0.1 M H2SO4 supporting electrolyte, at a controlled potential of −1.25 V for 40 seconds. The modified glassy carbon electrode (Ni−Pd/GCE) was conditioned in 0.5 M NaOH for about 50 cycles or more to obtain a reproducible voltammogram. The fabricated electrode was characterized using Cyclic Voltammetry (CV) and Chronoamperometry (CA). The results showed that the electrode had good electrocatalytic properties with respect to formaldehyde electrooxidation as a result of the synergistic effect of Ni and Pd nanoparticles combined with the glassy carbon technology. A sensitive oxidation peak for 1 mM formaldehyde was observed at about 0.43 V vs. Ag/AgCl/KCl (3 M) in 0.5 M NaOH, with a current density of 17 mA/cm2. It had a linear detection range from 10 μM to 1 mM (R=0.9985) and a detection limit of 5.4 μM. The electrode showed significant electrocatalytic activity towards the electrooxidation of formaldehyde in aqueous solution, was selective, reproducible and stable, hence can be used to detect formaldehyde at trace levels and can find application in fuel cells.
中文翻译:

镍钯基电化学传感器,用于甲醛的定量检测
甲醛是一种小的有机分子,尽管具有毒性,但在社会上具有广泛的用途。国际癌症研究机构(IARC)将甲醛归类为“已知致癌物”。甲醛电氧化由于其在燃料电池技术中的潜在应用以及由于其毒性而需要以痕量水平检测的要求,最近已成为人们关注的主要问题。甲醛电氧化已进行了许多研究,其中大多数由于吸附的中间体(如吸附的一氧化碳(COads)和甲酸(H 2))而受到电极钝化的影响。由甲醛的电氧化形成。在这项研究中,镍钯纳米粒子修饰的玻碳电极(Ni-Pd / GCE)被制备用于甲醛的电氧化。在0.1 MH 2 SO 4支持电解质中,将钯纳米粒子从2 mM PdCl 2在0.1 MH 2 SO 4支持电解质中电化学沉积到裸玻碳电极(GCE)上,持续240秒。镍纳米粒子从0.1 MH 2 SO 4中的0.5 M NiSO 4电化学沉积到PdGCE上支持电解质,在-1.25 V的受控电势下持续40秒。将改性玻璃碳电极(Ni-Pd / GCE)在0.5 M NaOH中处理约50个循环或更多,以获得可重现的伏安图。使用循环伏安法(CV)和计时电流法(CA)对制成的电极进行表征。结果表明,由于Ni和Pd纳米粒子协同玻璃碳技术的协同作用,该电极对甲醛的电氧化具有良好的电催化性能。在0.5 M NaOH中,相对于Ag / AgCl / KCl(3 M)在0.5 M NaOH中,在约0.43 V的电压下观察到1 mM甲醛的敏感氧化峰,电流密度为17 mA / cm 2。它的线性检测范围为10μM至1 mM(R = 0.9985),检测极限为5.4μM。该电极对水溶液中甲醛的电氧化表现出显着的电催化活性,具有选择性,可重现性和稳定性,因此可用于痕量甲醛检测,并可在燃料电池中找到应用。
更新日期:2018-01-09
中文翻译:

镍钯基电化学传感器,用于甲醛的定量检测
甲醛是一种小的有机分子,尽管具有毒性,但在社会上具有广泛的用途。国际癌症研究机构(IARC)将甲醛归类为“已知致癌物”。甲醛电氧化由于其在燃料电池技术中的潜在应用以及由于其毒性而需要以痕量水平检测的要求,最近已成为人们关注的主要问题。甲醛电氧化已进行了许多研究,其中大多数由于吸附的中间体(如吸附的一氧化碳(COads)和甲酸(H 2))而受到电极钝化的影响。由甲醛的电氧化形成。在这项研究中,镍钯纳米粒子修饰的玻碳电极(Ni-Pd / GCE)被制备用于甲醛的电氧化。在0.1 MH 2 SO 4支持电解质中,将钯纳米粒子从2 mM PdCl 2在0.1 MH 2 SO 4支持电解质中电化学沉积到裸玻碳电极(GCE)上,持续240秒。镍纳米粒子从0.1 MH 2 SO 4中的0.5 M NiSO 4电化学沉积到PdGCE上支持电解质,在-1.25 V的受控电势下持续40秒。将改性玻璃碳电极(Ni-Pd / GCE)在0.5 M NaOH中处理约50个循环或更多,以获得可重现的伏安图。使用循环伏安法(CV)和计时电流法(CA)对制成的电极进行表征。结果表明,由于Ni和Pd纳米粒子协同玻璃碳技术的协同作用,该电极对甲醛的电氧化具有良好的电催化性能。在0.5 M NaOH中,相对于Ag / AgCl / KCl(3 M)在0.5 M NaOH中,在约0.43 V的电压下观察到1 mM甲醛的敏感氧化峰,电流密度为17 mA / cm 2。它的线性检测范围为10μM至1 mM(R = 0.9985),检测极限为5.4μM。该电极对水溶液中甲醛的电氧化表现出显着的电催化活性,具有选择性,可重现性和稳定性,因此可用于痕量甲醛检测,并可在燃料电池中找到应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号