当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Straightforward Synthesis of Isoellipticine by Palladium‐Catalysed Coupling Reactions
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-01-09 , DOI: 10.1002/slct.201702602 Fabrício F. Naciuk 1, 2 , Joaquim A. M. Castro 1 , Bruno K. Serikava 1 , Paulo C. M. L. Miranda 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-01-09 , DOI: 10.1002/slct.201702602 Fabrício F. Naciuk 1, 2 , Joaquim A. M. Castro 1 , Bruno K. Serikava 1 , Paulo C. M. L. Miranda 1
Affiliation
|
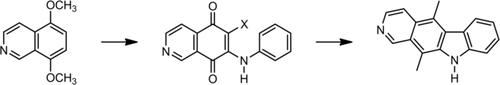
Our novel synthetic route to isoellipticine featured palladium‐catalyzed intramolecular reactions for the construction of the B ring of the pyridocarbazole nucleus. The adequate palladium‐catalyzed reaction depended upon the oxidation conditions that were applied in order to prepare the immediate synthetic precursor. When CAN was used to make the quinone intermediate, an oxidative cyclization through a double C−H bond activation was applied. Conversely, when the oxidation condition involved TCCA as oxidant, a direct C−H arylation was employed. Both approaches showed similar efficiencies in order to construct the pyridocarbazole nucleus. Isoellipticine was prepared in only 5 steps with a 21%–23% overall yield.
中文翻译:

钯催化偶联反应直接合成异玫瑰树碱
我们新型的异玫瑰树碱合成途径具有钯催化的分子内反应,可用于构建吡啶并咔唑核的B环。足够的钯催化反应取决于为制备直接合成前体所采用的氧化条件。当使用CAN制造醌中间体时,通过双CH键活化进行了氧化环化。相反,当氧化条件涉及TCCA作为氧化剂时,则采用直接的CH芳基化。两种方法显示出相似的效率,以构建吡啶并咔唑核。异玫瑰树碱仅分5步制备,总收率21%–23%。
更新日期:2018-01-09
中文翻译:

钯催化偶联反应直接合成异玫瑰树碱
我们新型的异玫瑰树碱合成途径具有钯催化的分子内反应,可用于构建吡啶并咔唑核的B环。足够的钯催化反应取决于为制备直接合成前体所采用的氧化条件。当使用CAN制造醌中间体时,通过双CH键活化进行了氧化环化。相反,当氧化条件涉及TCCA作为氧化剂时,则采用直接的CH芳基化。两种方法显示出相似的效率,以构建吡啶并咔唑核。异玫瑰树碱仅分5步制备,总收率21%–23%。









































 京公网安备 11010802027423号
京公网安备 11010802027423号