Chemosphere ( IF 8.1 ) Pub Date : 2018-01-09 , DOI: 10.1016/j.chemosphere.2018.01.031 Jifei Hou , Shasha Yang , Haiqin Wan , Heyun Fu , Xiaolei Qu , Zhaoyi Xu , Shourong Zheng
|
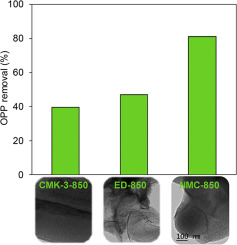
As a broad-spectrum preservative, toxic o-phenylphenol (OPP) was frequently detected in aquatic environments. In this study, N-doped mesoporous carbon was prepared by a hard template method using different nitrogen precursors and carbonization temperatures (i.e., 700, 850 and 1000 °C), and was used to activate peroxymonosulfate (PMS) for OPP degradation. For comparison, mesoporous carbon (CMK-3) was also prepared. Characterization results showed that the N-doped mesoporous carbon samples prepared under different conditions were perfect replica of their template. In comparison with ethylenediamine (EDA) and dicyandiamide (DCDA) as the precursor, N-doped mesoporous carbon prepared using EDA and carbon tetrachloride as the precursor displayed a higher catalytic activity for OPP degradation. Increasing carbonization temperature of N-doped mesoporous carbon led to decreased N content and increased graphitic N content at the expense of pyridinic and pyrrolic N. Electron paramagnetic resonance (EPR) analysis showed that PMS activation on N-doped mesoporous carbon resulted in highly active species and singlet oxygen, and catalytic PMS activation for OPP degradation followed a combined radical and nonradical reaction mechanism. Increasing PMS concentration enhanced OPP degradation, while OPP degradation rate was independent on initial OPP concentration. Furthermore, the dependency of OPP degradation on PMS concentration followed the Langmuir-Hinshelwood model, reflecting that the activation of adsorbed PMS was the rate controlling step. Based on the analysis by time-of-flight mass spectrometry, the degradation pathway of OPP was proposed.
中文翻译:

N掺杂介孔碳上高效催化过氧单硫酸盐活化以降解邻苯酚
作为一种广谱防腐剂,在水生环境中经常发现有毒的邻苯基苯酚(OPP)。在这项研究中,使用不同的氮前体和碳化温度(即:,700、850和1000°C),并用于活化过氧单硫酸盐(PMS)进行OPP降解。为了比较,还制备了中孔碳(CMK-3)。表征结果表明,在不同条件下制备的氮掺杂介孔碳样品是其模板的完美复制品。与乙二胺(EDA)和双氰胺(DCDA)作为前体相比,使用EDA和四氯化碳作为前体制备的N掺杂介孔碳对OPP降解表现出更高的催化活性。N掺杂介孔碳的碳化温度升高导致N含量降低和石墨N含量增加,但以吡啶二价和吡咯型N为代价。电子顺磁共振(EPR)分析表明,N掺杂介孔碳上的PMS活化导致高活性物质和单线态氧,而OPP降解的催化PMS活化遵循自由基和非自由基反应的联合机理。增加PMS浓度会增强OPP降解,而OPP降解速率与初始OPP浓度无关。此外,OPP降解对PMS浓度的依赖性遵循Langmuir-Hinshelwood模型,反映出吸附的PMS的活化是速率控制步骤。在飞行时间质谱分析的基础上,提出了OPP的降解途径。而OPP降解速率与初始OPP浓度无关。此外,OPP降解对PMS浓度的依赖性遵循Langmuir-Hinshelwood模型,反映出吸附的PMS的活化是速率控制步骤。在飞行时间质谱分析的基础上,提出了OPP的降解途径。而OPP降解速率与初始OPP浓度无关。此外,OPP降解对PMS浓度的依赖性遵循Langmuir-Hinshelwood模型,反映出吸附的PMS的活化是速率控制步骤。在飞行时间质谱分析的基础上,提出了OPP的降解途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号