Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2018-01-09 , DOI: 10.1016/j.cej.2018.01.016 Limin Hu , Guangshan Zhang , Meng Liu , Qiao Wang , Peng Wang
|
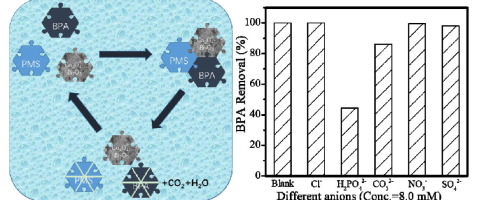
In this study, a novel Co3O4-Bi2O3 catalyst was synthesized using a microwave-assistant method, and the corresponding characteristics were studied through XRD, SEM, and N2 adsorption. As a peroxymonosulfate (PMS) activator, the catalytic activity of the synthesized catalyst was evaluated on Bisphenol A (BPA) removal, and the result showed that Co3O4-Bi2O3 catalyst promoted BPA degradation, where 100% BPA was degraded within 15 min under the condition of [catalyst] = 0.1 g·L−1 and [PMS]/[BPA]molar = 5. The Co3O4-Bi2O3/PMS system presented a good catalytic activity when the solution pH varied from 3.0 to 11.0. Under the different concentrations of various inorganic anions in the Co3O4-Bi2O3/PMS system, Cl− showed an inhibited effect at low concentration and a promoted effect at high concentration. CO32− showed a slight inhibited effect, while H2PO4− showed a considerable inhibited effect. Additionally, both NO3− and SO42− showed a negligible effect on BPA removal. For the real water bodies, both the drinking water and tap water showed a slight decrease in degradation efficiency of BPA but a distinctly negative effect on BPA mineralization, which might be contributed to the competition between BPA and indigenous organic matters in the two water matrices for their oxidation by radical species. The quenching experiment was enforced and it was found that both SO4− and
OH on the surface-bound of catalyst were the main active radicals in this system. Overall, the Co3O4-Bi2O3 catalyst as a PMS activator is a promising catalyst in PMS activation for sulfate radical-based wastewater treatment.
中文翻译:

过氧一硫酸盐与Co 3 O 4 -Bi 2 O 3催化剂活化作用增强的双酚A(BPA)降解:pH,无机阴离子和水基质的影响
本研究采用微波辅助法合成了新型的Co 3 O 4 -Bi 2 O 3催化剂,并通过XRD,SEM和N 2吸附研究了其相应的特性。作为过氧单硫酸盐(PMS)活化剂,对合成催化剂的催化活性进行了双酚A(BPA)去除评估,结果表明,Co 3 O 4 -Bi 2 O 3催化剂促进了BPA的降解,其中100%的BPA降解了在[催化剂] = 0.1 g·L -1和[PMS] / [BPA]摩尔 = 5的条件下,在15分钟内。Co 3 O 4当溶液的pH从3.0变化至11.0时,-Bi 2 O 3 / PMS体系表现出良好的催化活性。下中的CO的不同浓度的各种无机阴离子的3 ö 4 -Bi 2 ö 3 / PMS系统,氯-表明在低浓度的抑制效果,并在高浓度的促进效果。CO 3 2-呈小幅抑制效应功能,H 2 PO 4 -表明有相当抑制效果。此外,这两种NO 3 -和SO 4 2-对去除双酚A的影响可忽略不计。对于真实的水体,饮用水和自来水都显示出BPA降解效率略有下降,但对BPA矿化有明显的负面影响,这可能是导致BPA与两种水基质中本地有机物之间竞争的原因。它们被自由基种氧化。淬火实验被实施,并且发现这两个SO 4 -和
OH的表面结合催化剂的分别为在该系统中的主要活性基团。总体而言,作为PMS活化剂的Co 3 O 4 -Bi 2 O 3催化剂是用于硫酸根基废水处理的PMS活化中很有希望的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号