当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Synthesis of Key Propargylic Alcohol Intermediates of the Anti-HIV Drug Efavirenz
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00376 Yu Zheng 1 , Lilu Zhang 1 , Eric Meggers 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00376 Yu Zheng 1 , Lilu Zhang 1 , Eric Meggers 1
Affiliation
|
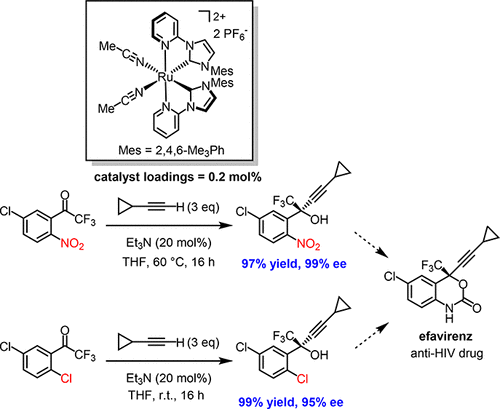
The catalytic, enantioselective synthesis of key propargylic alcohol intermediates toward the synthesis of the anti-HIV drug efavirenz is reported. Using a recently reported chiral-at-ruthenium catalyst ( J. Am. Chem. Soc. 2017, 139, 4322), catalytic enantioselective alkynylations of 1-(2,5-dichlorophenyl)-2,2,2-trifluoroethanone (99% yield, 95% ee) and 1-(5-chloro-2-nitrophenyl)-2,2,2-trifluoroethanone (97% yield, 99% ee) are achieved using catalyst loadings of merely 0.2 mol % (ca. 500 TON).
中文翻译:

抗HIV药物依非韦伦的关键炔丙基醇中间体的催化对映选择性合成
据报道,关键的炔丙醇中间体对抗HIV药物依非韦伦的合成具有催化,对映选择性。使用最近报道手性在钌催化剂(J.化学会会志。 2017,139,4322),1-(2,5-二氯苯基)-2,2,2-三氟乙酮的对映选择性催化alkynylations(99%使用仅0.2 mol%(约500 TON)的催化剂负载量即可实现收率95%ee)和1-(5-氯-2-硝基苯基)-2,2,2-三氟乙酮(97%收率99%ee) )。
更新日期:2018-01-09
中文翻译:

抗HIV药物依非韦伦的关键炔丙基醇中间体的催化对映选择性合成
据报道,关键的炔丙醇中间体对抗HIV药物依非韦伦的合成具有催化,对映选择性。使用最近报道手性在钌催化剂(J.化学会会志。 2017,139,4322),1-(2,5-二氯苯基)-2,2,2-三氟乙酮的对映选择性催化alkynylations(99%使用仅0.2 mol%(约500 TON)的催化剂负载量即可实现收率95%ee)和1-(5-氯-2-硝基苯基)-2,2,2-三氟乙酮(97%收率99%ee) )。











































 京公网安备 11010802027423号
京公网安备 11010802027423号