Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Scalable Synthesis Pathway to Nanoporous Metal Structures
ACS Nano ( IF 15.8 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acsnano.7b06667 Christopher Coaty 1 , Hongyao Zhou 1 , Haodong Liu 1 , Ping Liu 1
ACS Nano ( IF 15.8 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acsnano.7b06667 Christopher Coaty 1 , Hongyao Zhou 1 , Haodong Liu 1 , Ping Liu 1
Affiliation
|
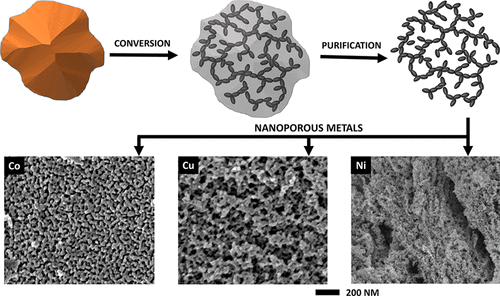
A variety of nanoporous transition metals, Fe, Co, Au, Cu, and others, have been readily formed by a scalable, room-temperature synthesis process. Metal halide compounds are reacted with organolithium reductants in a nonpolar solvent to form metal/lithium halide nanocomposites. The lithium halide is then dissolved out of the nanocomposite with a common organic solvent, leaving behind a continuous, three-dimensional network of metal filaments that form a nanoporous structure. This approach is applicable to both noble metals (Cu, Au, Ag) and less-noble transition metals (Co, Fe, Ni). The microstructures of these nanoporous transition metals are tunable, as controlling the formation of the metal structure in the nanocomposite dictates the final metal structure. Microscopy studies and nitrogen adsorption analysis show these materials form pores ranging from 2 to 50 nm with specific surface areas from 1.0 m2/g to 160 m2/g. Our analysis also shows that pore size, pore volume, and filament size of the nanoporous metal networks depend on the mobility of target metal and the amount of lithium halide produced by the conversion reaction. Further, it has been demonstrated that hybrid nanoporous structures of two or more metals could be synthesized by performing the same process on mixtures of precursor compounds. Metals (e.g., Co and Cu) have been found to stabilize each other in nanoporous forms, resulting in smaller pore sizes and higher surface areas than each element in their pure forms. This scalable and versatile synthesis pathway greatly expands our access to additional compositions and microstructures of nanoporous metals.
中文翻译:

纳米多孔金属结构的可扩展合成途径
通过可扩展的室温合成工艺,很容易形成各种纳米多孔过渡金属,Fe,Co,Au,Cu等。在非极性溶剂中,金属卤化物与有机锂还原剂反应形成金属/卤化锂纳米复合材料。然后用常见的有机溶剂将卤化锂从纳米复合材料中溶解出来,留下连续的三维金属丝网络,形成纳米孔结构。此方法适用于贵金属(Cu,Au,Ag)和次贵金属过渡金属(Co,Fe,Ni)。这些纳米多孔过渡金属的微结构是可调的,因为控制纳米复合物中金属结构的形成决定了最终的金属结构。2 / g至160 m 2 / g。我们的分析还表明,纳米孔金属网络的孔径,孔体积和长丝尺寸取决于目标金属的迁移率和转化反应产生的卤化锂的量。此外,已经证明可以通过对前体化合物的混合物进行相同的过程来合成两种或更多种金属的杂化纳米孔结构。已经发现金属(例如,Co和Cu)以纳米孔形式彼此稳定,从而导致比其纯净形式的每种元素更小的孔径和更大的表面积。这种可扩展且用途广泛的合成途径极大地扩展了我们对纳米多孔金属的其他成分和微观结构的访问范围。
更新日期:2018-01-11
中文翻译:

纳米多孔金属结构的可扩展合成途径
通过可扩展的室温合成工艺,很容易形成各种纳米多孔过渡金属,Fe,Co,Au,Cu等。在非极性溶剂中,金属卤化物与有机锂还原剂反应形成金属/卤化锂纳米复合材料。然后用常见的有机溶剂将卤化锂从纳米复合材料中溶解出来,留下连续的三维金属丝网络,形成纳米孔结构。此方法适用于贵金属(Cu,Au,Ag)和次贵金属过渡金属(Co,Fe,Ni)。这些纳米多孔过渡金属的微结构是可调的,因为控制纳米复合物中金属结构的形成决定了最终的金属结构。2 / g至160 m 2 / g。我们的分析还表明,纳米孔金属网络的孔径,孔体积和长丝尺寸取决于目标金属的迁移率和转化反应产生的卤化锂的量。此外,已经证明可以通过对前体化合物的混合物进行相同的过程来合成两种或更多种金属的杂化纳米孔结构。已经发现金属(例如,Co和Cu)以纳米孔形式彼此稳定,从而导致比其纯净形式的每种元素更小的孔径和更大的表面积。这种可扩展且用途广泛的合成途径极大地扩展了我们对纳米多孔金属的其他成分和微观结构的访问范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号