当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of the Caged Indole Alkaloid Arboridinine Enabled by aza-Prins and Metal-Mediated Cyclizations
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b07724 Pei Gan 1 , Jennifer Pitzen 1 , Pei Qu 1 , Scott A. Snyder 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b07724 Pei Gan 1 , Jennifer Pitzen 1 , Pei Qu 1 , Scott A. Snyder 1
Affiliation
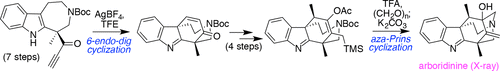
|
Although alkaloid natural products possess incredible diversity when considered broadly, certain domains are sometimes shared by several members, even from different sub-collections. Such homology can point to potential synthetic strategies. Herein, we highlight how such an analysis of the natural product arboridinine pinpointed two key elements of structural similarity that suggested the value of a metal-mediated 6-endo-dig cyclization to fashion its tetracyclic indolenine core, as well as the need to develop what could be considered a reversed polarity aza-Prins cyclization to deliver its tertiary allylic alcohol and final cage structure. The power of the latter design element is highlighted by several failures in achieving similar functional group patterning through more traditional aza-Prins and Mannich cyclization strategies. Overall, these operations fueled an inaugural 13-step racemic synthesis of the target; exploration of varied solutions for the enantioselective preparation of a key 7-membered indole-containing piece afforded a 16-step formal asymmetric solution.
中文翻译:

氮杂-Prins和金属介导的环化使笼养吲哚生物碱Arboridinine的全合成
尽管从广义上考虑,生物碱天然产物具有令人难以置信的多样性,但某些领域有时由几个成员共享,甚至来自不同的子集合。这种同源性可以指向潜在的合成策略。在此,我们强调对天然产物阿波瑞丁的这种分析如何确定结构相似性的两个关键要素,这些要素表明金属介导的 6-endo-dig 环化对形成其四环假吲哚核心的价值,以及开发什么的必要性可以被认为是反极性 aza-Prins 环化以提供其叔烯丙醇和最终的笼状结构。通过更传统的 aza-Prins 和 Mannich 环化策略在实现类似功能组模式方面的几次失败突出了后一种设计元素的力量。全面的,这些操作推动了目标的首次 13 步外消旋合成;探索用于对映选择性制备关键 7 元含吲哚片的各种解决方案,提供了 16 步形式的不对称解决方案。
更新日期:2018-01-08
中文翻译:

氮杂-Prins和金属介导的环化使笼养吲哚生物碱Arboridinine的全合成
尽管从广义上考虑,生物碱天然产物具有令人难以置信的多样性,但某些领域有时由几个成员共享,甚至来自不同的子集合。这种同源性可以指向潜在的合成策略。在此,我们强调对天然产物阿波瑞丁的这种分析如何确定结构相似性的两个关键要素,这些要素表明金属介导的 6-endo-dig 环化对形成其四环假吲哚核心的价值,以及开发什么的必要性可以被认为是反极性 aza-Prins 环化以提供其叔烯丙醇和最终的笼状结构。通过更传统的 aza-Prins 和 Mannich 环化策略在实现类似功能组模式方面的几次失败突出了后一种设计元素的力量。全面的,这些操作推动了目标的首次 13 步外消旋合成;探索用于对映选择性制备关键 7 元含吲哚片的各种解决方案,提供了 16 步形式的不对称解决方案。



























 京公网安备 11010802027423号
京公网安备 11010802027423号