当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Non-Canonical Proximal Heme Ligand Affords an Efficient Peroxidase in a Globin Fold
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b12621 Moritz Pott 1 , Takahiro Hayashi 1 , Takahiro Mori 1 , Peer R E Mittl 2 , Anthony P Green 3 , Donald Hilvert 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b12621 Moritz Pott 1 , Takahiro Hayashi 1 , Takahiro Mori 1 , Peer R E Mittl 2 , Anthony P Green 3 , Donald Hilvert 1
Affiliation
|
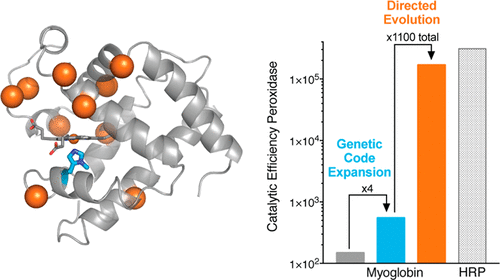
Expanding the range of genetically encoded metal coordination environments accessible within tunable protein scaffolds presents excellent opportunities for the creation of metalloenzymes with augmented properties and novel activities. Here, we demonstrate that installation of a noncanonical Nδ-methyl histidine (NMH) as the proximal heme ligand in the oxygen binding protein myoglobin (Mb) leads to substantial increases in heme redox potential and promiscuous peroxidase activity. Structural characterization of this catalytically modified myoglobin variant (Mb NMH) revealed significant changes in the proximal pocket, including alterations to hydrogen-bonding interactions involving the prosthetic porphyrin cofactor. Further optimization of Mb NMH via a combination of rational modification and several rounds of laboratory evolution afforded efficient peroxidase biocatalysts within a globin fold, with activities comparable to those displayed by nature's peroxidases.
中文翻译:

非典型近端血红素配体在珠蛋白折叠中提供有效的过氧化物酶
扩大可调谐蛋白质支架内可访问的基因编码金属配位环境的范围,为创造具有增强特性和新活性的金属酶提供了绝佳的机会。在这里,我们证明了在氧结合蛋白肌红蛋白 (Mb) 中安装非常规 Nδ-甲基组氨酸 (NMH) 作为近端血红素配体导致血红素氧化还原电位和混杂过氧化物酶活性的显着增加。这种催化修饰的肌红蛋白变体 (Mb NMH) 的结构表征揭示了近端口袋的显着变化,包括涉及假体卟啉辅因子的氢键相互作用的改变。
更新日期:2018-01-08
中文翻译:

非典型近端血红素配体在珠蛋白折叠中提供有效的过氧化物酶
扩大可调谐蛋白质支架内可访问的基因编码金属配位环境的范围,为创造具有增强特性和新活性的金属酶提供了绝佳的机会。在这里,我们证明了在氧结合蛋白肌红蛋白 (Mb) 中安装非常规 Nδ-甲基组氨酸 (NMH) 作为近端血红素配体导致血红素氧化还原电位和混杂过氧化物酶活性的显着增加。这种催化修饰的肌红蛋白变体 (Mb NMH) 的结构表征揭示了近端口袋的显着变化,包括涉及假体卟啉辅因子的氢键相互作用的改变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号