Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2018-01-08 , DOI: 10.1016/j.jcis.2018.01.029 H. Ashassi-Sorkhabi , S. Abolghasemi-Fakhri , B. Rezaei Moghadam , H. Javan
|
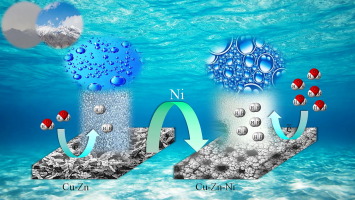
An efficient and non-precious metal catalyst is a key factor for hydrogen evolution reaction (HER). Here we report that the fabrication of highly ordered porous arrays of Cu-Zn-Ni alloy has been carried out in a one-step electrochemical route at a constant apparent current density of −3 A·cm−2. The optimum film composition and reactivity of the electrodes for catalytic hydrogen evolution reaction were analyzed by using different current densities, deposition time and bath concentration. For this purpose, onset potentials in linear sweep voltammograms (LSV) were compared. The structure and morphology of nanoporous Cu-Zn-Ni and Cu-Zn alloy were characterized by SEM and energy dispersive X-ray (EDS) analysis.
The experimental results on the behavior of electrocatalytic activity of prepared alloys showed that the addition of nickel to the alloys improves of the electrocatalytic performance of the electrodes toward HER. In addition, enhancement of electrochemical activity toward hydrogen evolution can be attributed to the large electrochemical active surface area and porous structure of Cu-Zn-Ni alloy. In order to improvement of reaction kinetics, Tafel plots were derived from LSV voltammograms, and the exchange current densities for HER on synthesized electrodes (Cu-Zn and Cu-Zn-Ni alloys) were calculated about 3.2 × 10−5 and 2.1 × 10−3 mA·cm−2, respectively.
中文翻译:

一种制备圆柱形纳米多孔结构的高度有序阵列的电化学途径及其对高效氢释放的电催化性能
高效且非贵金属催化剂是析氢反应(HER)的关键因素。在这里,我们报告说,Cu-Zn-Ni合金的高度有序多孔阵列的制造已在一步法电化学路线中以-3 A·cm -2的恒定视在电流密度进行。通过使用不同的电流密度,沉积时间和浴液浓度,分析了用于催化氢逸出反应的电极的最佳膜组成和反应性。为此,比较了线性扫描伏安图(LSV)中的起始电位。通过SEM和能量色散X射线(EDS)分析表征了纳米多孔Cu-Zn-Ni和Cu-Zn合金的结构和形貌。
对所制备合金的电催化活性行为的实验结果表明,向合金中添加镍可改善电极对HER的电催化性能。另外,对氢析出的电化学活性增强可以归因于Cu-Zn-Ni合金的大电化学活性表面积和多孔结构。为了改善反应动力学,从LSV伏安图得出Tafel图,并计算了合成电极(Cu-Zn和Cu-Zn-Ni合金)上HER的交换电流密度,约为3.2×10 -5和2.1×10 -3 mA·cm -2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号