当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Contribution of Coulomb Interactions to a Two-Step Crystal Structure Phase Transformation Coupled with a Significant Change in Spin Crossover Behavior for a Series of Charged FeII Complexes from 2,6-Bis(2-methylthiazol-4-yl)pyridine
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02721 Kazuyuki Takahashi 1 , Mitsunobu Okai 1 , Tomoyuki Mochida 1 , Takahiro Sakurai 2 , Hitoshi Ohta 3 , Takashi Yamamoto 4 , Yasuaki Einaga 4 , Yoshihito Shiota 5 , Kazunari Yoshizawa 5 , Hisashi Konaka 6 , Akito Sasaki 6
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02721 Kazuyuki Takahashi 1 , Mitsunobu Okai 1 , Tomoyuki Mochida 1 , Takahiro Sakurai 2 , Hitoshi Ohta 3 , Takashi Yamamoto 4 , Yasuaki Einaga 4 , Yoshihito Shiota 5 , Kazunari Yoshizawa 5 , Hisashi Konaka 6 , Akito Sasaki 6
Affiliation
|
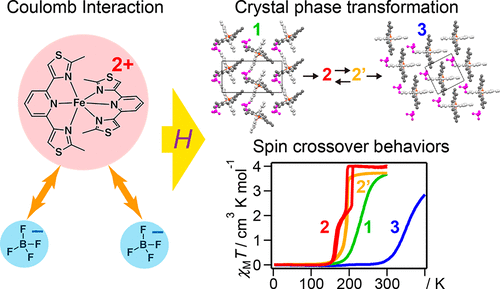
A series of [FeII(L)2](BF4)2 compounds were structurally and physically characterized (L = 2,6-bis(2-methylthiazol-4-yl)pyridine). A crystal structure phase transformation from dihydrate compound 1 to anhydrous compound 3 through partially hydrated compounds 2 and 2′ upon dehydration was found. Compounds 1 and 3 exhibited a gradual spin crossover (SCO) conversion, whereas compounds 2 and 2′ demonstrated two-step and one-step abrupt SCO transitions, respectively. An X-ray single-crystal structural analysis revealed that one-dimensional and two-dimensional Fe cation networks linked by π stacking and sulfur–sulfur interactions were formed in 1 and 3, respectively. A thermodynamic analysis of the magnetic susceptibility for 1, 2′, and 3 suggests that the enthalpy differences may govern SCO transition behaviors in the polymorphic compounds 2′ and 3. A structural comparison between 1 and 3 indicates that the SCO behavior variations and crystal structure transformation in the present [FeII(L)2](BF4)2 compounds can be interpreted by the relationship between the lattice enthalpies mainly arising from Coulomb interactions between the Fe cations and BF4 anions as in typical ionic crystals.
中文翻译:

库仑相互作用对两步晶体结构相变的贡献,以及自2,6-双(2-甲基噻唑-4-基)吡啶的一系列带电Fe II配合物的自旋交叉行为的显着变化
对一系列[Fe II(L)2 ](BF 4)2化合物进行结构和物理表征(L = 2,6-双(2-甲基噻唑-4-基)吡啶)。发现在脱水时通过部分水合的化合物2和2'从二水合物化合物1到无水化合物3的晶体结构相变。化合物1和3表现出逐渐的自旋交叉(SCO)转化,而化合物2和2'分别展示了两步和一步的SCO过渡。X射线单晶结构分析表明,在1和3中分别形成了由π堆积和硫-硫相互作用连接的一维和二维Fe阳离子网络。磁化率为热力学分析1,2' ,和3表明,焓差可以管理SCO变行为的多晶型化合物中的2'和3。1和3之间的结构比较表明,目前的[FeII(L)2 ](BF 4)2化合物可以通过晶格焓之间的关系来解释,这些晶格焓主要由典型的离子晶体中的Fe阳离子和BF 4阴离子之间的库仑相互作用引起。
更新日期:2018-01-08
中文翻译:

库仑相互作用对两步晶体结构相变的贡献,以及自2,6-双(2-甲基噻唑-4-基)吡啶的一系列带电Fe II配合物的自旋交叉行为的显着变化
对一系列[Fe II(L)2 ](BF 4)2化合物进行结构和物理表征(L = 2,6-双(2-甲基噻唑-4-基)吡啶)。发现在脱水时通过部分水合的化合物2和2'从二水合物化合物1到无水化合物3的晶体结构相变。化合物1和3表现出逐渐的自旋交叉(SCO)转化,而化合物2和2'分别展示了两步和一步的SCO过渡。X射线单晶结构分析表明,在1和3中分别形成了由π堆积和硫-硫相互作用连接的一维和二维Fe阳离子网络。磁化率为热力学分析1,2' ,和3表明,焓差可以管理SCO变行为的多晶型化合物中的2'和3。1和3之间的结构比较表明,目前的[FeII(L)2 ](BF 4)2化合物可以通过晶格焓之间的关系来解释,这些晶格焓主要由典型的离子晶体中的Fe阳离子和BF 4阴离子之间的库仑相互作用引起。











































 京公网安备 11010802027423号
京公网安备 11010802027423号